ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sneha P. Tenpe1 and D. V. Parwate2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Citrus reticulata (mandarin) plant parts and soil have been opted for elemental evaluation by INAA with comparator and k0 method. Two reference material Soil-7 (IAEA) and apple leaves (SRM1515) were used as standards. Sodium was estimated by irradiation of samples in Am-Be source followed by counting on NaI(Tl) detector. Samples were also irradiated in KAMINI reactor, IGCAR, Kalpakkam and radioactive assay was carried out using HPGe detector coupled with 5MCA. Concentration of Na, Al, Mn, Ca, Fe, Sr, Sm, Sc, La, Th, Br, K, Zn, Ce were determined by NAA and in all 15 radicals were identified by XRF for same samples. It has been seen that soil with certain mineralogical and chemical make-up behaves respectively for availability of micronutrients
Keywords |
| INAA, XRF, Citrus reticulate-orange |
INTRODUCTION |
| Orange is fruit of Citrus genus of flowering plant in the rue family Rutaceae. While the origin of citrus fruits cannot be precisely identified, researchers believe that the fruit began to appear in Southeast Asia at least 4000BC. From there, these slowly spread. Worldwide trade in citrus fruits didn't appear until the 19th century and trade in orange juice developed as late as 1940 [1]. The recent research indicates plant origin in Australia, New Caledonia and New Guinea, yet some researchers believe that the origin is in the part of South East Asia bordered by Northeast India, Burma (Myanmar) and Yunnan province of China. The mandarin orange (Citrus reticulata) which is a native of China and South-eastern Asia, was not taken to Europe during ancient and medieval times but was known and extensively planted in China and Japan at early date [1]. It is in this region some commercial species such as oranges, mandarins and lemons originally appeared. In terms of international trade Citrus fruits have the highest value as there are two main markets of Citrus fruit via, fresh fruit market and processed Citrus fruit market (mainly orange juice). 2010 figures published by the UN’s Food and Agriculture Organization ranks India third in the world for oranges (6268100 metric tons), behind only Brazil and the United States . In India, mandarin and sweet orange production is concentrated in Andhra Pradesh, Maharashtra, Karnataka, Punjab, Haryana and Rajasthan. Among the various states of India, Maharashtra gives highest yield regardless of that stands behind Madhya Pradesh, Assam, West Bengal and Nagaland in productivity per unit area. Growing and potential belts in Maharashtra include Nagpur, Akola, Amravati and Wardha. Nagpur orange is a variety of orange (Mandarin- Citrus reticulata) which gives city its pseudonym "Orange City". |
| There is consistent growing demand of the consumers for Nagpur oranges and the fruit quality as well. Citrus necessitate sixteen essential elements for normal growth, production and for fruit quality. As studied, both deficiency and excess of nutrients lead to diminution in crop yield, coupled with inferior fruit quality. The deficiency symptoms are observed in leaves, wilting of parts or all canopy like leaf drop, twig dieback, abnormal flowering, general canopy decline, growth of new shoot with flowering and reduced size of fruit. Nutrient deficiencies can be caused by leaching due to excessive irrigation or rain, naturally low soil nutrient levels, soil nutrient imbalances, improper pH or insufficient or incorrect fertilizer application [2, 3]. However, nitrogen, zinc, magnesium and iron deficiencies are common and correctable. |
| Citrus does not tolerate salinity as well. For this reason, most Citrus grows poorly in coastal and atoll environments. High levels of salt in water will increase the osmotic pressure and reduce the ease of water uptake by trees. It is believed that at suitable dosages the rare earth elements (REEs) can improve growth and productivity as well as plant resistance to adverse conditions. REEs are used as fertilizers mainly by Chinese agriculturists. The beneficial effects are associated with stimulus on nutrient uptake by plants and increase synthesis of chlorophyll. The recommended application of REEs is dependent on their bioavailability in soils, which in turn mainly depends on the exchangeable fraction of REEs and moreover strongly affected by the physicochemical properties of soil [4-6]. |
| Sub-optimum nutrition of zinc is globally one of the prime concerns of targeting sustainability in Citrus production. Citrus apparently has difficulty in absorbing sufficient Zn from many soils and anything that affects the root system adversely, is likely to reduce the Zn-intake remarkably. With root injury and other mineral deficiencies that may have a pronounced effect on Zn-deficiency too[7] Global occurrence of Zn-deficiency known by various names like rosette, little leaf, mottle leaf etc. is basically characterized by interveinal chlorosis which may or may not be coupled with rosetting or small leaves. Zinc deficiency may also appear due to competition from P, Fe, Mn, Ca and up to some extent K, besides a number of soil properties influencing the Zn-availability. Similarly magnesium and copper deficiencies both reduce the development of root system, when they are under acute shortage. |
| Such relationships are commonly found when another deficiency is acting as a limiting factor. Fruit production is considerably lower under acute deficiency conditions. Researcher found that there were differences between elemental composition of healthy and infected citrus leaves. Even the mass fractions of REEs were found decreased consistently in citrus variegated chlorosis diseased leaves [8]. This would also explain the nutritional disorder suggested by some researchers, even though only few studies about trace elements can be found in the literature [9]. In this work, instrumental neutron activation analysis (INAA) and X-ray fluorescence (XRF) technique have been applied to investigate elemental concentration of Citrus orchard soil and plant parts around Nagpur. |
MATERIAL AND METHODS |
| INAA and XRF are two nuclear analytical techniques employed in this study, both allow qualitative as well as quantitative analysis. There are many conspicuous feature of these techniques like non-destructive, has high precision and accuracy, has simultaneous multielement capacity. The techniques are extremely versatile for applications in many fields of science, research and quality control has low detection limits, and a large dynamic range of concentrations. |
| INAA: Neutron activation analysis (NAA) allows incoherent sampling of elements as it disregards the chemical form of a sample and focus only on its nucleus. The method is based on bombardment of sample with neutron or a charged particle which leads to activation of isotopes by (n,γ) reaction. The induced radioactivity of the newly formed radioactive isotope is measured by NaI (Tl) scintillation or HPGe detector. As the radioactive emission or decay path for each element is well studied, depending on the literature it is possible to study the γ emission spectra of the radioactive sample and determine the concentration of element within it. This is general principle of NAA however, depending on the sample type, element of interest and desired nuclide to be analysed have different approach and practiced. When the analysis performed without any chemical treatment the process is called INAA. There are also different methods for calculating the elemental concentration depending on measurement of radioactivity. Two methods used were comparator or relative method and k0 or single comparator method. In the comparator method mass fraction of sample (m) is calculated using |
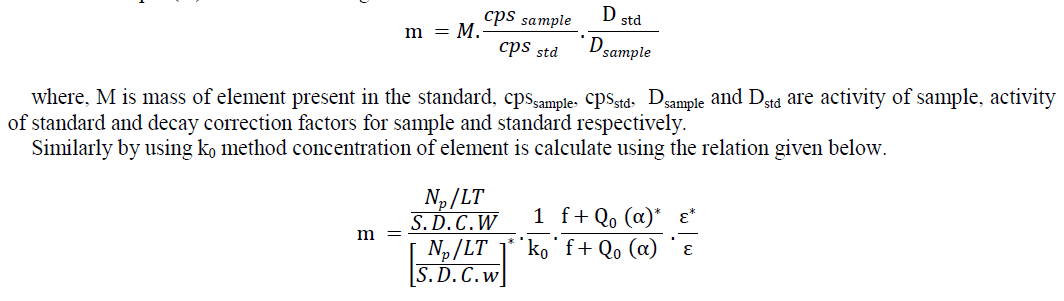 |
| where, W and w are masses of sample and single comparator respectively, LT is live time for counting, k0 and Q0 are nuclear constant, f is sub-cadmium to epi-thermal neutron flux ratio, α is epi-thermal neutron flux shape factor and ε is relative efficiency of detector[10]. |
| XRF: X ray fluorescence is the emission of characteristic "secondary" X ray from a sample that has been excited by bombarding with high energy X rays or gamma rays. Each element has element orbits of characteristic energy. When an energetic photon provided by a primary radiation source remove an inner electron, an electron from outer shell drops into its place. These transitions yield a fluorescent photon with characteristic energy equal to difference in energy of initial and final orbital. The wavelength of this fluorescent radiation can be calculated from Plank’s law- |
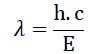 |
| Fluorescent radiation can be analysed either by sorting the energies of photon or by separating the wavelength of the radiation. After separation the intensity of each characteristics radiation is directly related to the amount of each element in the sample. Proportional counter used as detector where an incoming X ray photon leads to ionisation with the amount of charge produced being proportional to the energy of the incoming photon[11]. |
EXPERIMENTAL |
| The geographical indication was applied for Nagpur Orange with the registrar of GIs in India and effective as of April 2014. So far the uptakes of elemental composition from the raw product soil of Mandarin around Nagpur have not been reported. Samples of various parts of orange tree and soil from fruit farm Kandala, Katol, Nagpur were collected and analyzed for minor and trace elements by INAA. The selected farm conducts the orange production in the conventional manner since 20 years, using artificial soluble fertilizer (NPK) and organic fertilizer in every alternate year with apt pesticides. In the field for sampling, plants were selected on the basis of distribution similarly, the plant nearby water resource (well) and drainage during rainy season are also re-evaluated for studying the distribution of sodium. The groves which are uninfected, healthy and well grown were selected for sampling. Approximately 5 months old leaves were taken from the periphery of fruiting twigs at shoulder height in the month of November-December. Leaves samples were collected with a downward pull, away from the bud located in the leaf axils so the petioles remain attached to the leaves. Along with that stem and fruits were also collected in the middle of the season and soil samples were collected from about 15-20 cm below the surface. |
| The plant samples were thoroughly washed with distilled water to remove dust or resuspended soil particles adhered to the surface. These were dried and converted to powder (100mesh). Ash was prepared by in muffle furnace maintained at ~500-550 0C and one-tenth of weight reduction was observed in this process. Reference materials Apple leaves (SRM 1515) and Soil-7 (IAEA) were used as comparator standards. 50-60 mg of the ash and reference material were weighed and heat sealed in polythene bags. |
| The set of four samples and standards were irradiated for 72 hours in Am-Be source having flux-103 n.cm-2 s-1.The samples were placed on the paraffin wax block of 1 cm thickness so as to get thermal neutrons. Counting was done on NaI(Tl) detector (with background correction) having resolution about 9-10% using 8K MCA and software PHAST. Sodium in plant and soil samples was determined by comparator method [12]. |
| Likewise sets of three samples along with standards, blank and Au foil were irradiated for short (5min) and long time (6 hours) in KAMINI reactor at IGCAR, Kalpakkam. At power supply of 20 kW in pneumatic fast transfer system position having flux- 1012 n.cm-2 s-1, f-value 24 ±0.5, α value -0.04±0.004[13]. Short-lived activities were measured using HPGe detector with 5K MCA in FBTR at reactor site. Long irradiated samples were transferred to Radiochemistry Laboratory, in a lead bottle and after the cooling time the γ-activity was measured on HPGe detector. Element mass fractions were calculated by comparator and k0 method with software package APTEC. The concentration of element was calculated by using comparator and k0 relation. Specific count rate is the peak area corrected for saturation, decay during cooling and counting periods are normalized to the unit weight of the elements. |
RESULTS AND DISCUSSION |
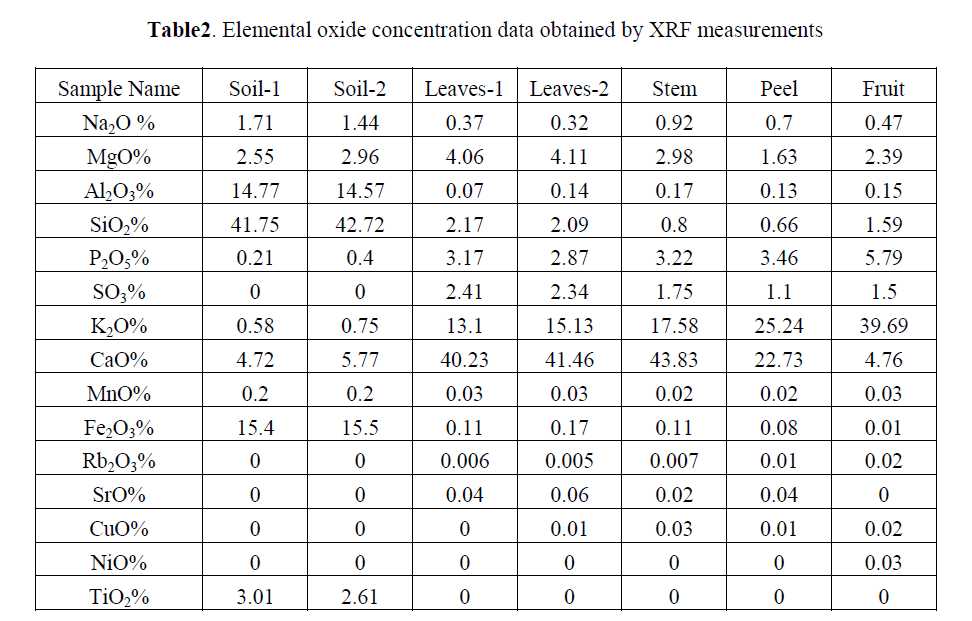
| We have determined the elements Na, Al, Mn, Ca, Fe, Sr, Sm, Sc, La, Th, Br, K, Zn and Ce in the Citrus reticulata. Elemental concentration in soil and plant ash samples of Citrus reticulata (Mandarin) including mean and standard deviation are listed in Table1. The f and t tests were applied on results, obtained by comparator and k0 method at 95% confidence level. There was no statistical difference found in the precision of the two methods. |
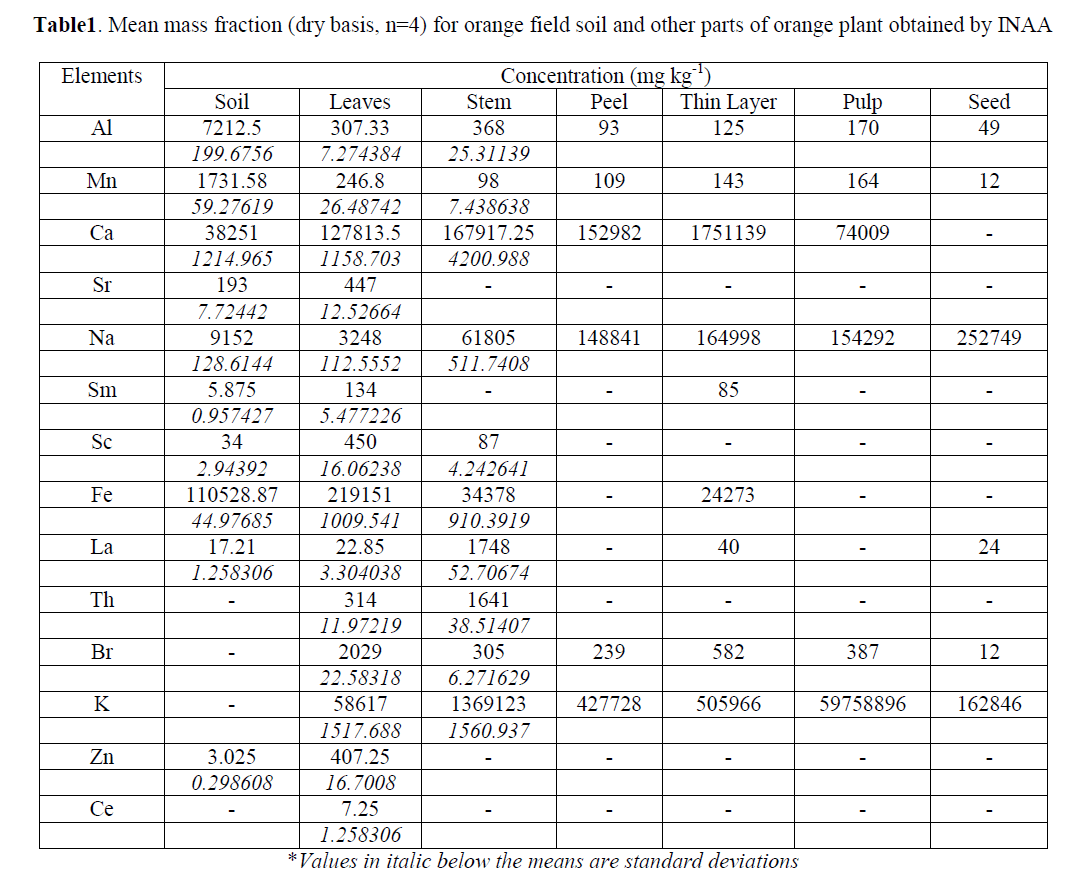 |
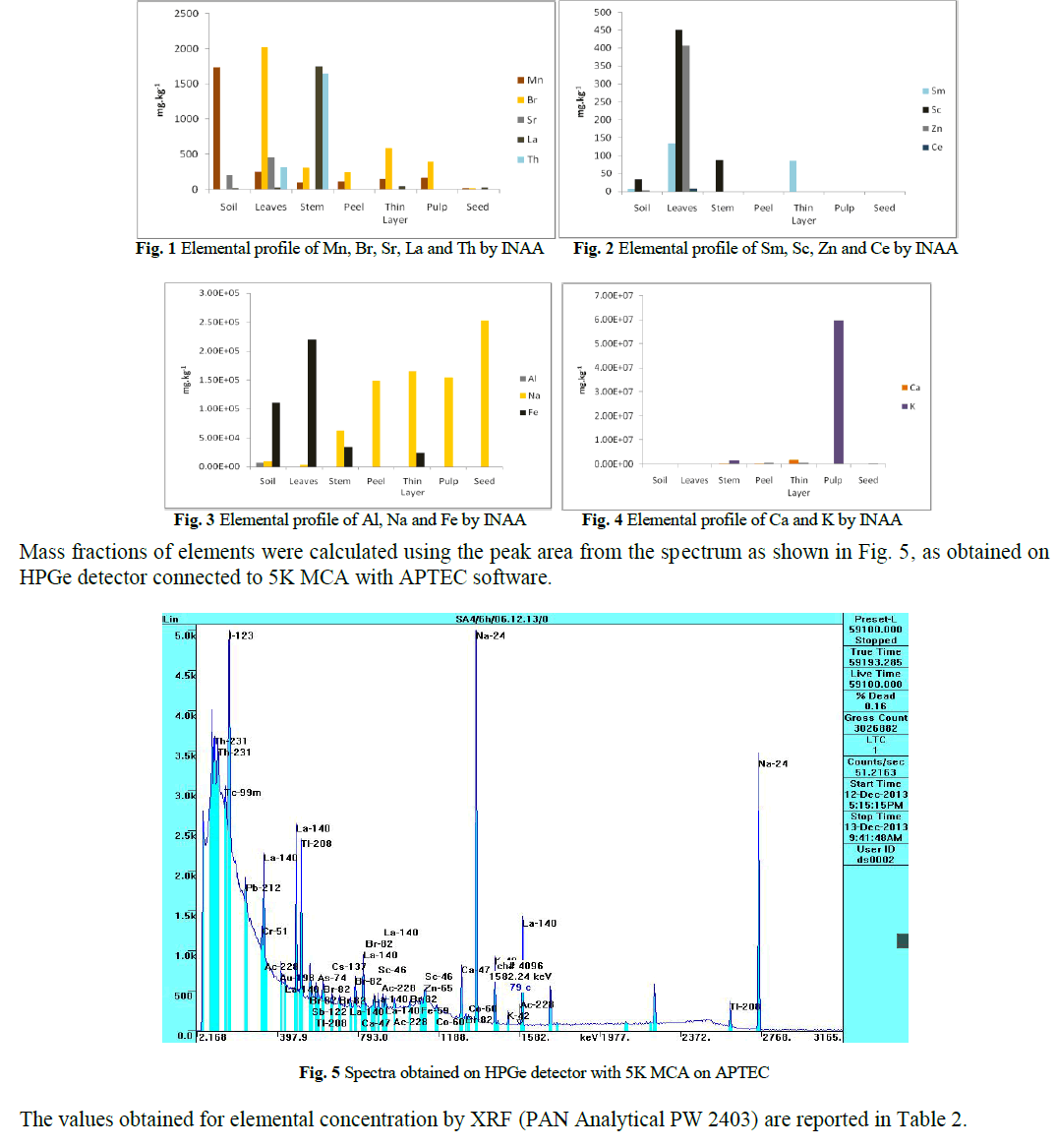
| The mass fractions of raw material soil and different parts of plant were compared. Elemental profiles were drawn for minor, trace and rare element content obtained by INAA in soil, leaves, stem, peel, thin layer (white thin layer present casing pulp), pulp and seed as shown in Fig. 1 to 4. Leaves have been found to contain maximum amount of Fe and Al. Whereas Na is present higher quantity in seed and K was present in large excess in the fruit pulp. |
 |
 |
| The ability of orange plant to uptake essential nutrients, various trace elements and transfer from soil to upper parts has been reported [14, 15]. We have also observed the uptake comprehension co-relation from soil to leaves and other parts of plant for the elements like Al, Mn, Ca, Na, Fe, La and K. Significant differences were found in the soil and the leave samples. Correlation factor for trace elements contained show large seasonal variation [16]. Analysis also comprised with the REEs like Sm, La, Sc and Ce as previous study has demonstrated that Citrus plants are accumulators of REEs. Such affinity for REEs may indicate that these elements play a physiological role in Citrus plants. The amount of RREs in agricultural supplies is largely variable. The highest mass fraction of REE found is in fertilizers containing phosphate, although few provide more REE than recommended dose. Such high input can significantly increase the content of REE in soil, which may cause harmful effects to environment and human. The most important threats arising from the increased input of REEs in the environment continue to be in countries with naturally high REE abundance levels in soil. Close monitoring needed in countries where phosphate based fertilizers mined from monazite deposits are used in area where soil conditions are favourable to REE mobility, availability and uptake plants or at sites located near landfills where surface runoff could contaminate the local environment. |
| Na was estimated for the several plants, though all the samples were collected from the same field but located in different surrounding and was with different water supply level. A perusal of data shows that Na present in the soil and leaves near by the water source and drainage water stream during rainy season was found elevated. Not much variation is observed in Fe. Regardless of species, the concentration of sodium in leaves was higher in the plant exhibiting damage than the plant appearing healthy. It appears that sodium in soil results in the alkalinity of soil. Higher concentration of sodium can also affect the species in ways other than direct toxicity by sodium [17]. The Na in soil can reduce the soil structure and can have adverse effect on microenvironment of the rhizosphere by reducing oxygen to the roots and causing puddling of fine textured soil. Large variation in concentration for sodium may be due to different surroundings. The drainage water may carry sodium salt with the flow in rainy season, which on water evaporation tends to augment sodium in nearby soil and consequently to the plants. |
| During previous study of Citrus orchards of Assam, Meghalaya, Tripura and Mizoram of north-east India indicated large scale Fe-induced Zn-deficiency and both were negatively correlated with each other. In the present work similar circumstance was found in area nearby Nagpur. The soil was found to contain Fe in excess which leads to Zn deficiency which may lead to chlorosis of oranges caused by antagonistic effect of Fe on Zn availability. Chlorosis of sweet orange was mainly due to Zn-deficiency caused by antagonistic effect of Fe on Zn-availability. Accumulation of Zn can occur before visible wilting or reduced water transport [7]. Zinc levels fluctuate seasonally in the wood of diseased and healthy trees. Seasonal cycles have been reported for Zn levels in the outer trunk wood of both blighted and healthy trees with increased levels in the winter and reduced levels in the summer. |
CONCLUSION |
| From the results obtained it can be concluded that, it is necessary to streamline the processes controlling elements availability to plants in order to improve the technologies for their efficient management. To compensate the loss due to deficiency of elemental concentration it is important to study the availability in soil and its uptake under different soil and climatic conditions. This will be helpful for management to take decision under different Citrus cropping systems and ease up the understanding of micronutrients. Soil fertility indicates the relation to varying levels of fruit yield. For improving the quality of fruit with variation in elemental uptake of Citrus species with different climatic condition future research could be directed towards seasonal variation of elemental concentration on Citrus reticulata and few other Citrus species around Nagpur. |
ACKNOWLEDGEMENTS |
| The authors are thankful to IGCAR, Kalpakkam and IBM, Nagpur for co-ordinating and providing the necessary facilities. Thanks are due to University Grant Commission, New Delhi for providing grant for this project work (F.No. 39-790/2010(SR) dt. 11 Jan 2011). |
References |
|