Excipients used in the Formulation of Tablets
Karthik Varma V*
Department of Pharmaceutical Analysis, Vikas college of pharmaceutical Sciences, Jawaharlal Nehru technological University, Suryapet, Nalgonda, Telangana, India
- *Corresponding Author:
- Karthik Varma V
Department of Pharmaceutical Analysis, Vikas college of pharmaceutical Sciences
Jawaharlal Nehru technological University, Suryapet, Nalgonda, Telangana, India
E-mail: karthikvarma145@gmail.com
Received date: 07/07/2016; Accepted date: 31/07/2016; Revised date: 26/07/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
Abstract
Excipients are additive substances used in tablet formulation to improve bulkiness, disintegration, dissolution rate and bioavailability of the drug. The drug and excipient interaction study is carried using Infrared Spectrum to know the stability of excipients and drug.
Keywords
Excipients; Bioavailability; Diluents: Binders; Super disintegrants
Introduction
Excipients are inert substances used as diluents or vehicles for a drug. In the pharmaceutical industry it is a catch-all term which includes various sub-groups comprising diluents or fillers [1-9], binders or adhesives, disintegrants, lubricants, glidant, flavors, colors and sweeteners. All of these must meet certain criteria as follows [10-20]:
a) Physiologically inert.
b) Acceptable to regulatory agencies.
c) Physiologically and chemically stable.
d) Free from bacteria.
e) Should not interfere with the bioavailability of the drug.
f) Commercially available in the form and purity commensurate to pharmaceutical standards.
g) Low cost, inexpensive.
h) Meet the standards of regulatory requirements.
To assure that no excipient interferences with the utilization of the drug, the formulator must carefully and critically evaluate combinations of the drug with each of the contemplated excipients and must as certain compliance of each ingredient with existing standards and regulations. The screening of drug-excipient and excipient-excipient interactions carried out in pre formulation studies [21-30].
List of Excipients [31-40]
Diluents: Diluents are fillers used to make up the volume of tablet if tablet is inadequate to produce the volume. Diluents used as disintegrants in dispersible and orally disintegrating tablet.
Example: Lactose, Spray dried lactose, Micro crystalline cellulose (Avicel 101 and 102), Pvpk30 (Pearlitol SD200 and 25C), Sorbitol, Dibasic calcium phosphate dehydrate, Calcium sulphate dehydrate etc.
Binders: Binders are used as binding agent in tablets; it provides cohesive strength to powdered materials. Binders are added in both dry and wet form to form granules.
Example: Gelatin, glucose, Lactose, cellulose derivatives-Methyl cellulose, Ethyl cellulose, Hydroxy propylmethyl cellulose,Hydroxy propyl cellulose, starch, Poly vinyl pyrrolidone (Povidone), Sodium alginate, Carboxymethylcellulose, Acacia etc.
Lubricants: Used to reduce the friction between die wall and tablet, prevent adhesion of tablet to dies and punches. Helps in easy ejection of tablets from die cavity. Classified in to 2 types.
Example: Insoluble- Stearic acid, Magnesium stearate, Calcium stearate, Talc, Paraffin.
Soluble- Sodium lauryl sulphate, Sodium benzoate, PEG 400, 600,8000 etc.
Glidants: Helps in free flowing of granules from hopper to die cavity. Minimize friction between particles.
Example: Colloidal Silicon dioxide (Aerosil), Cornstarch, Talc etc.
Anti-adherents: These are added to prevent adhesion of tablet material to punches and dies.
Example: Talc
Anti-adherent: Prevent sticking of tablet to dies and punches.
Superdisintegrants: When they come in contact with water in oral cavity/GIT break down in to small particles.
Example: Croscarmellose sodium (Ac-di-sol),Crospovidone (Polyplasdone), and Sodium starch glycollate, Starch etc.
Role of Super disintegrants in the manufacturing of tablets
Disintegrating agents are substances included in tablet formulations and in some hard shell capsule formulations to promote moisture penetration and dispersion of the matrix of the dosage form in dissolution fluids. An oral solid dosage form should ideally disperse into the primary particles from which it was prepared. Although various compounds have been proposed and evaluated as disintegrants, relatively few are in common usage today. Traditionally, starch has been the disintegrant of choice in tablet formulations, and it is still widely used. For instance, starch generally has to be present at levels greater than 5% to adversely affect compactibility, especially in direct compression. Moreover, intra granular starch in wet granulations is not as effective as dry starch [41-50].
Characteristics of disintegrant
The ideal disintegrant should have the following characteristics:
• Poor solubility
• Poor gel formation
• Good hydration capacity
• Good compressibility and flow properties
• No tendency to form complexes with the drugs
Factors affecting action of disintegrants
• Percentage of disintegrants present in the tablets.
• Types of substances present in the tablets.
• Combination of disintegrants.
• Presence of surfactants.
• Hardness of the tablets.
• Nature of Drug substances.
• Mixing and Screening.
Classification of Superdisintegrants
1. Modified starches (Sodium starch glycolate, NF)
Description: Sodium carboxy methyl starch; the carboxy methyl groups induces hydrophilicity and cross-linking reduces solubility.
Trade name: Explotab®(Edward Mendell Co.), Primojel® Generichem Corp.), Tablo® (Blanver, Brazil)
2. Modified cellulose (Croscarmellose, NF)
Description: Sodium carboxymethyl cellulose which has been cross-linked to render the material insoluble.
Trade name: AcDiSol® (FMC Corp.), Nymcel ZSX® (Nyma, Netherlands), Primellose® (Avebe, Netherlands)
3. Cross-linked poly-vinyl pyrrolidone (Crospovidone, NF)
Description: Cross-linked poly vinyl pyrrolidone; cross-linking render the material insoluble in water.
Trade name: Crospovidone M® (BASF Corp.), Kollidon CL® (BASF Corp.)
4. Excipient profiles
PVP K30
Synonyms: E1201; Kollidon; Plasdone; poly [1-(2-oxo-1-pyrrolidinyl)ethylene]; polyvidone; polyvinylpyrrolidone; PVP; 1-vinyl-2-pyrrolidinone polymer.
Chemical Name and CAS Registry Number: 1-Ethenyl-2-pyrrolidinone homopolymer (9003-39-8)
Empirical Formula: (C6H9NO)n
Structural Formula:
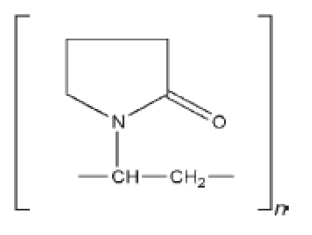
Functional Category: Disintegrant; dissolution aid; suspending agent; tablet binder.
Applications:
Binders in wet granulation processes, coating agents suspending, stabilizing, or viscosity-increasing agent.
Typical Properties:
Acidity/alkalinity: pH = 3.0–7.0 (5% w/v aqueous solution).
Density (bulk): 0.29–0.39 g/cm3 for Plasdone.
Density (tapped): 0.39–0.54 g/cm3 for Plasdone.
Density (true): 1.180 g/cm3
Flowability: 20 g/s for povidone K-15;16 g/s for povidone K-29/32.
Melting point: Softens at 1500C.
Moisture content: Hygroscopic.
Solubility
Freely soluble in acids, chloroform, ethanol (95%), ketones, methanol, and water; practically insoluble in ether, hydrocarbons, and mineral oil. In water, the concentration of a solution is limited only by the viscosity of the resulting solution, which is a function of the K-value.
Viscosity (dynamic)
The viscosity of aqueous povidone solutions depends on both the concentration and the molecular weight of the polymer employed. Viscosity (dynamic) is 5.5–8.5
Stability and Storage Conditions
Povidone darkens to some extent on heating at 1508C, with a reduction in aqueous solubility. It is stable to a short cycle of heat exposure around 110–1308C.
Incompatibilities
Povidone is compatible in solution with a wide range of inorganic salts, natural and synthetic resins, and other chemicals [51-60].
Crosscarmellose Sodium [61-70]
Synonyms: AC-Di-sol, cross linked carboxyl methylcellulose sodium, explocel.
2. Description: It is odourless, white or greyish white powder.
3. Functional category: Tablet and capsule disintegrant.
4. Solubility: Insoluble in water.
5. Bulk density: 0.529 glcm3
6. Tapped density: 0.819 glcm3
7. Incompatibilities: Efficacy of crosscarmellose sodium may be slightly reduced in tablet formulations contain hygroscopic excipients such as sorbital.
8. Pharmaceutical applications: Disintengrant for capsules tablets & granules. In capsules it is used as a disintegrant in the concentration of 10 to 25% and tablets in the concentration of 0.5 to 5.0%.
Crospovidone [71-75]
1. Synonyms: cross linked povidone, kollidon CL, polyvinyl pyrrolidone
2. Structural Formula
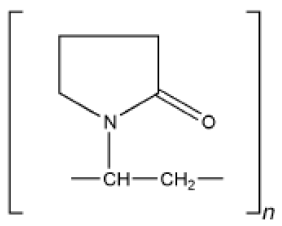
3. Description: It is a white to creamy white, finely divided, free flowing practically taste less, odorless or nearly odorless hygroscopic powder
4. Functional category: Tablet disintegrants.
5. pH: 5.0 to 8.0.
6. Density: 1.22 gm/ cm3
7. Solubility: Practically insoluble in water and most common organic solvents.
8. Stability and storage conditions: Since crospovidone is hygroscopic, it should be stored in an air tight container in a cool, dry place.
9. Incompatibilities: Organic and inorganic pharmaceutical ingredients. When exposed to high water level, cross povidone may form molecular adduct with some materials.
10. Pharmaceutical applications: solubility enhancer [61-65].
Sodium Starch Glycolate [76-85]
1. Synonyms: Sodium carboxy methyl starch, explotab, primojel
2. Description: It is white to off – white, odorless, tasteless, free flowing powder. It consists of oval or spherical granules.
3. Functional category: Tablet and capsule disintegrant
4. Solubility: Insoluble in organic solvents. Sparingly soluble in ethanol.
5. pH: 5.5 to 7.5
6. Stability and storage conditions: It is stable and it should be stored in well closed container to protect it from wide variations in humidity and temperature that may cause caking.
7. Pharmaceutical applications: oral pharmaceuticals as a disintegrant in capsule and tablet formulations in the concentration of 2 to 8%.
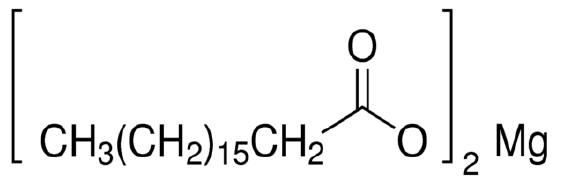
Magnesium Stearate [86-90]
1. Synonyms: Metallic stearate, Magnesium Salt.
2. Structural Formula: [CH3(CH2)16COO]2Mg
3. Description: Fine white, precipitated or milled, impalatable powder of low bulk density. Odor and taste are slight but characteristic. The powder is unctuous and readily adheres to skin.
4. Functional Category: Tablet or Capsule lubricant.
5. Solubility: Practically insoluble in water, alchohol, ethers. Slightly soluble in warm benzene and warm ethanol (95%).
6. Melting Point: 117°C to150°C
7. Stability and storage conditions: Stable and should be stored in a cool, dry place in a well closed container.
8. Incompatibilities: strong acids, alkalis and iron salts, strong oxidizing materials. Magnesium Stearate cannot be used in products containing aspirin, some vitamins and most alkaloidal salts.
9. Pharmaceutical applications: cosmetics, foods and pharmaceutical formulations. It is primarily used as a lubricant in capsule and tablet manufacture at concentration between 0.25 – 0.5% w/w.
Aerosil [91-99]
1. Synonyms: Colloidal silica, aerosil, fumed silica, light anhydrous silicic acid.
2. Description: It is submicroscopic, light, loose, bluish-white, odorless, tasteless, non-gritty, amorphous powder.
3. Functional category: Glidant, suspending and / or viscosity increasing agent, anticaking agent.
4. PH: 3.3-4.4 (1 in 25 aqueous dispersion)
5. Solubility: Insoluble in purified water forms a colloidal dispersion. Soluble in hot solutions of alkali hydroxide. Insoluble in acids, except hydrofluoric acid.
6. Stability and storage conditions: Colloidal Silicon Dioxide is Hygroscopic, but absorbs large quantities of water without liquefying store in a well closed container.
7. Incompatibilities: Incompatible with diethylstilbestrol preparations.
8. Pharmaceutical applications:
• Drying agent for hygroscopic materials. Absorbent dispersing agent for liquids in powders or suppositories.
• Glidant and antiadherent in tabletting processes and encapsulation (0.1 – 0.5%)
• Thixotropic thickening and suspending agent in gels and semisolid preparations in the concentration of 2-10%.
• Emulsion stabilizer in the concentration of 1-5%.
• At the concentration of 0.5 to 2%, it is used in aerosols to promote particulate suspension.
Conclusion
This review may supply precious knowledge regarding the excipients which are additive substances used in tablet formulation to improve bulkiness, disintegration, dissolution rate and bioavailability of the drug.
Acknowledgement
This content of the article is scrutinized and approved by M. Murali and written by Karthikvarma V
References
- Patel P, Patel P, Giram P (2015) Bioanalytical Method Development and Validation for Latanoprost Quantification in Pharmaceutical Opthalmic Microemulsion Formulation by RP-HPLC. J Anal Bioanal Tech 6:284.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Characterization of Physical, Thermal and Spectral Properties of Biofield Treated 2, 6-Diaminopyridine. J Develop Drugs 4:133.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Physical, Thermal and Spectral Properties of Biofield Energy Treated 2,4-Dihydroxybenzophenone. Clin Pharmacol Biopharm 4:145.
- Kharshoum RM, Aboutaleb HA (2016) Formulation, Development and Evaluation of Meclozine Hydrochloride Microspheres. J Bioequiv Availab 8:027-032.
- Samanta A, Bandyopadhyay B, Das N (2016) Formulation of Catechin Hydrate Nanocapsule and Study of its Bioavailability. Med chem (Los Angeles) 6:399-404.
- Patel BD, Chhalotiya UK, Patel DB (2016) Quantification of Newer Anti-Cancer Drug Clofarabine in their Bulk and Pharmaceutical Dosage Form. J Chromatogr Sep Tech 7:328.
- Kojima S, Mori T, Shibata T, Kobayashi Y (2015) Broadband Terahertz Time-Domain and Low-Frequency Raman Spectroscopy of Crystalline and Glassy Pharmaceuticals. Pharm Anal Acta 6:401.
- Jethara SI, Patel MR (2015) Enhanced Solubility and Dissolution Rate of Aceclofenac by Using Spray Drying Techniques. Intel Prop Rights 3:140.
- Sreelakshmy V, Deepa MK, Mridula P (2016) Green Synthesis of Silver Nanoparticles from Glycyrrhiza glabra Root Extract for the Treatment of Gastric Ulcer. J Develop Drugs 5:152.
- Montasser MS, Younes AM, Hegazi MM, Dashti NH, El-Sharkawey AE, et al. (2016) A Novel Eco-friendly Method of Using Red Algae (Laurencia papillosa) to Synthesize Gold Nanoprisms. J Nanomed Nanotechnol 7:383.
- Naranis K (2014) Separation of Gas and Vapour using Ethylene-Octane Polymer Membranes. J Bioproces Biotechniq 4:169.
- Dana E, Ardestani SS, Khodabandehlo H (2015) The Effects of Tannic Acid on Some Properties of Cow Gelatin's Film. J Food Process Technol 6:491.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Physical, Thermal and Spectral Properties of Biofield Treated 1,2,3-Trimethoxybenzene. J Develop Drugs 4:136.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Characterization of Physical, Thermal and Spectral Properties of Biofield Treated 2, 6-Diaminopyridine. J Develop Drugs 4:133.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Singh R, et al. (2015) Physical, Thermal and Spectroscopic Studies on Biofield Treated p-Dichlorobenzene. Biochem Anal Biochem 4:204.
- Trivedi MK, Patil S, Mishra RK, Jana S (2015) Thermal and Physical Properties of Biofield Treated Bile Salt and Proteose Peptone. J Anal Bioanal Tech 6:256.
- Jethara SI, Patel MR (2015) Enhanced Solubility and Dissolution Rate of Aceclofenac by Using Spray Drying Techniques. Intel Prop Rights 3:140.
- 18. Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Biofield Treatment: An Effective Strategy for Modulating the Physical and Thermal Properties of O-Nitrophenol, M-Nitrophenol and P-Tertiary Butyl Phenol. J Bioanal Biomed 07:156-163.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al.(2015) Characterization of Physical, Thermal and Spectral Properties of Biofield Treated O-Aminophenol. Pharm Anal Acta 6:425.
- Leiva GE, Šegatin N, Mazzobre MF, Abramovic H, Abram V, et al. (2015) Multi-analytical Approach to Oxidative Stability of Unrefined Argan, Chia, Rosa Mosqueta and Olive Oils. J Nutr Food Sci 6:450.
- Carlquist JF, McKinney JT, Horne BD, Camp NJ, Cannon-Albright L, et al. (2011) Common Variants in 6 Lipid-Related Genes Discovered by High-Resolution DNA Melting Analysis and Their Association with Plasma Lipids. J Clinic Experiment Cardiol 2:138.
- Peter Amala Sujith A, Hymavathi TV, Yasoda Devi P (2012) A Study on the Different Methods of Preparation of Lutein from Supercritical Fluid Processed Lutein Esters. J Nutr Food Sci 2:154.
- Figueiras A, Cardoso O, Veiga F, Carvalho RBF, Ballaro G (2015) Preparation and characterization of Trimethoprim inclusion complex with Methyl-β-Cyclodextrin and determination of its antimicrobial activity. Pharm Anal Acta 6:405.
- Zhang L, Zhao H, Zhou G, Niu T, Yang J (2010) Simulation Database System of the Active Ingredients in Compound Decoction of Chinese Medicine. J Bioequiv Availab 2: 131-134.
- Naveed S, Sajid S (2016) Degradation in Pharmaceutical Creams: Ascorbic Acid Demonstrating Phenomenon: A Review. J Bioequiv Availab 8:080-083.
- Khokhlov AL, Shitov LN, Ryska M, Dzhurko YA, Kubeš V, et al. (2016)The Pharmacokinetic Properties and Bioequivalence of Methyldopa Formulations:Results of an Open-label, Randomized, Two-period, Crossover, Single-dose Study. J Bioequiv Availab 8:185-190.
- Delsin SD, Mercurio DG, Fossa MM, Maia Campos PMBG (2015) Clinical Efficacy of Dermocosmetic Formulations Containing Spirulina Extract on Young and Mature Skin: Effects on the Skin Hydrolipidic Barrier and Structural Properties. Clin Pharmacol Biopharm 4:144.
- Galicia-Quintanar C, Valle-Laisequilla CF, Soto-Molina H, Lara-Padilla E, Juan Huerta-Cruz C, et al. (2015) Adverse Events Reactions Reported With the Use of a Fixed-Dose Combination of Nor- Pseudoephedrine, Triiodothyronine, Atropine, Aloin and Diazepam in Obese Mexican Patients. J Pharmacovigil 3:185.
- Karwa M, Arora S, Agrawal SG (2013) Recent Regulatory Amendment in Schedule Y: Impact on Bioequivalence Studies Conducted In India. J Bioequiv Availab 5:174-176.
- Xie PS, Sun S, Xu S, Guo L (2014) Value the Unique Merit of HPTLC Image Analysis and Extending its Performance by Digitalization for Herbal Medicines Quality Control. J Chromatograph Separat Techniq 5:249.
- Singh A, Tandon S, Sand NK. (2014) Active Ingredient Estimation of Clopyralid Formulation by Reversed Phase HPLC. J Chromatogr Sep Tech 6:257.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Mishra R (2015) Influence of Biofield Treatment on Physicochemical Properties of Hydroxyethyl Cellulose and Hydroxypropyl Cellulose. J Mol Pharm Org Process Res 3: 126.
- Jain R, Sukla SK, Nema N, Panday A (2015) Drug Nano-particle: A Release Kinetics. J Nanomed Nanotechnol 6:317. doi:10.4172/2157-7439.
- Chauhan MK, Bhatt N (2015) A Simple and Modified Method Development of Vancomycin Using High Performance Liquid Chromatography. J Chromatogr Sep Tech 6:296.
- Mohd AB, Vemula SK (2016) Formulation and Pharmacokinetics of Vitamin E TPGS Melt Dispersion Granules: An Approach to Improve Oral Delivery of Flurbiprofen. J Bioequiv Availab 8:089-94.
- Mukthinuthalapati MA, Bukkapatnam V (2015) A Novel Validated Stability- Indicating RP-HPLC Method for the Determination of Exemestane (Steroidal Aromatase Inhibitor). J Bioequiv Availab 7:288-292. doi:10.4172/jbb.1000256
- Jana S, Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Characterization of Physicochemical and Thermal Properties of Chitosan and Sodium Alginate after Biofield Treatment. Pharm Anal Acta 6:430.
- Patel P, Patel P, Giram P (2015) Bioanalytical Method Development and Validation for Latanoprost Quantification in Pharmaceutical Opthalmic Microemulsion Formulation by RP-HPLC. J Anal Bioanal Tech 6:284.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Characterization of Physical, Thermal and Spectral Properties of Biofield Treated 2, 6-Diaminopyridine. J Develop Drugs 4:133.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Physical, Thermal and Spectral Properties of Biofield Energy Treated 2,4-Dihydroxybenzophenone. Clin Pharmacol Biopharm 4:145.
- Enose AA, Dasan P, Sivaramakrishanan H, Kakkar V (2016) Formulation, Characterization and Pharmacokinetic Evaluation of Telmisartan Solid Dispersions. J Mol Pharm Org Process Res 4:131.
- Maroof K, Zafar F, Ali H, Naveed S, Tanwir S (2016) Scope of Nanotechnology in Drug Delivery. J Bioequiv Availab 8:001-005.
- Mohd AB, Vemula SK (2016) Formulation and Pharmacokinetics of Vitamin E TPGS Melt Dispersion Granules: An Approach to Improve Oral Delivery of Flurbiprofen. J Bioequiv Availab 8:089-94.
- Krishnaiah YSR (2010) Pharmaceutical Technologies for Enhancing Oral Bioavailability of Poorly Soluble Drugs. J Bioequiv Availab 2: 028-036.
- Abdul Althaf S, Sailaja PB, Ashwin Kumar M (2012) Formulation, Evaluation and Mathematical Modelling of Clopidogrel Bisulphate & Aspirin Immediate Release Bilayer Tablets. Pharmaceut Anal Acta 3:194.
- Hart ML, Do DP, Ansari RA, Rizvi SAA (2013) Brief Overview of Various Approaches to Enhance Drug Solubility. J Develop Drugs 2: 115.
- Jethara SI, Patel MR (2015) Enhanced Solubility and Dissolution Rate of Aceclofenac by Using Spray Drying Techniques. Intel Prop Rights 3:140.
- Cavallari C, Fini A, Pérez-Artacho Santos B (2013) Thermal Study of Anhydrous and Hydrated Forms of Olanzapine. Pharm Anal Acta 4:237.
- Ertekin B, Çimen Z, Yilmaz H, Yilmaz UT (2016) Synthesis and Characterization of Polyaniline/Ignimbrite Nano-Composite Material. J Material Sci Eng 5: 237.
- Samanta A, Bandyopadhyay B, Das N (2016) Formulation of Catechin Hydrate Nanocapsule and Study of its Bioavailability. Med chem (Los Angeles) 6:399-404.
- Su K, Yang Y, Wu Q, Mao Y, Hu Y (2016) Preparation of Polymeric Micelles of Curcumin with Pluronic P123 and Assessment of Efficacy against B16 Cells In vitro. Adv Pharmacoepidemiol Drug Saf 5:202.
- Shakeel F, Ramadan W, Shafiq S (2009) Solubility and Dissolution Improvement of Aceclofenac using Different Nanocarriers. J Bioequiv Availab 1: 039-043.
- González-Montiel S, Baca-Téllez S, Martínez-Otero D, Álvarez- Hernández A, Cruz-Borbolla J, et al. (2015) Crystal Structure and Hirshfeld Surface Analysis of 1,2-Bis((2-(Bromomethyl)Phenyl)Thio)Ethane and Two Polymorphs of 1,2-Bis((2-((Pyridin-2-ylthio)Methyl)Phenyl)Thio)Ethane. Mod Chem appl 3:154.
- Hossain MF, Amoyaw PNA, Saluja HS, Khan FMO (2016) Evaluation of the Physicochemical Properties of a Novel Antimalarial Drug Lead, Cyclen Bisquinoline. Mod Chem appl 4: 181.
- Hart ML, Do DP, Ansari RA, Rizvi SAA (2013) Brief Overview of Various Approaches to Enhance Drug Solubility. J Develop Drugs 2: 115.
- Higuchi T, Arnold RD, Tucker SJ, Busse LW. The physics of tablet compression. I. A preliminary report. J Am Pharm Assoc, 41(2):93-96, 1952.
- Rankell AS, Higuchi T., Physics of tablet compression. XV. Thermodynamic and kinetic aspects of adhesion under pressure. J Pharm Sci, 57(4): 574-577, 1968.
- Parikh DM (Ed)., Handbook of pharmaceutical granulation technology (Vol. 198). Informa Health Care, 2010. This Journal is © IPEC-Americas Inc March 2014 J. Excipients and Food Chem. 5 (1) 2014 - 3
- Reier GE, Shangraw RF., Microcrystalline cellulose in tableting. J Pharm Sci, 55(5):510-514, 1966.
- Nachaegari SK, Bansal AK., Coprocessed excipients for solid dosage forms. Pharmaceutical Technology, 28(1):52-65,
- Vervaet C, Remon JP., Melt granulation. In Parikh, DM (Ed), Handbook of pharmaceutical granulation technology (Vol. 198). Informa Health Care, pp. 435- 448, 2010.
- Lakshman JP, Kowalski J, Vasanthavada M, Tong WQ, Joshi YM, Serajuddin ATM., Application of melt granulation technology to enhance tableting properties of poorly compactible high dose drugs. J Pharm Sci, 100(4):1553-1565, 2011.
- Vasanthavada M, Wang Y, Haefele T, Lakshman JP, Mone M, Tong W, Joshi YM, Serajuddin ATM., Application of melt granulation technology using twin screw extruder in development of high dose modified release tablet formulation. J Pharm Sci, 100(5):1923- 1934.
- Dalziel G, Nauka E, Zhang F, Kothari S, Xie M., Assessment of granulation technologies for an API with poor physical properties. Drug Dev Ind Pharm, 39(7):985-995, 2013.
- Politis SN, Rekkas DM., The evolution of the manufacturing science and the pharmaceutical industry. Pharm Res, 28(7), 1779-1781, 2011.
- Kowalski J, Lakshman J P, Serajuddin ATM, Tong WQ, Vasanthavada M., Continuous process for making pharmaceutical compositions. U.S. Patent US20110037185 A1 (2011).
- Moreton RC., Pharmaceutical excipients – the continuing paradox(es) of formulation science. J Excip Food Chem, 4(4):107-110, 2013.
- Shah AC and Mlodozeniec AB, (1977), Mechanisms of surface lubrication: Influence of duration of lubricant excipient mixing on processing characteristics of powders and properties of compressed tablets. J. Pharm. Sci., 66, (10), 1377 – 1382.
- Tan SB, Moreton RC and Smith D, (1979), Flow properties and tablet weight uniformity: Effects of granule size and machine speed, British Pharmaceutical Conference 1979, Exeter, UK, (J. Pharm. Pharmacol., 31, (Suppl.), 74P).
- Frattini C and Simioni L, (1984), Should magnesium stearate be assessed in the formulation of solid dosage forms be weight or by surface area? Drug Devel. Ind. Pharm., 10, (7), 1117 – 1130.
- Bolhuis G.K. and Lerk CF., (1977), Film forming of tablet lubricants during the mixing process of solids. Acta Pharm. Technol., 23, (1), 13-20.
- Bavitz JF and Shiromani PK, (1986), Granulation surface area as a basis for magnesium stearate concentration in tablet formulations, Drug Devel. Ind. Pharm., 12, (14), 2481 – 2492.
- Ahmed HA, (2010), Personal communication.
- Van Veen B, Pajander J, Zuurman K, Lappalainen R, Poso A, Frijlink HW and Ketolainen J, (2005), The effect of powder blend and tablet structure on drug release mechanisms of hydrophobic starch acetate matrix tablets, Eur. J. Pharm. Biopharm., 61, 149 – 157.
- Elversson J, Andersson K, Millqvist-Fureby A, (2007), An Atomic Force Microscopy Approach for Assessment of Particle Density Applied to Single Spray-Dried Carbohydrate Particles, J. Pharm. Sci., 96, (4), 905 – 912.
- Sun CC, (2008), True density of microcrystalline cellulose, J. Pharm. Sci., 94 (10), 2132 – 2134.
- Moreton RC, (2009), Functionality and Performance of Excipients in a Quality-by-Design World. Excipient Variability, QbD and Robust Formulations, Am. Pharm. Rev., 12, (2), 24 – 27.
- Guidance for Industry: Immediate Release Solid Oral Dosage Forms: Scale-up and Post-approval Changes: Chemistry, Manufacturing and Controls, in Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation, US FDA, Center for Drug Evaluation and Research, November 1995.
- Abdul S, Poddar SS. A flexible technology for modified release of drugs:multi layered tablets. Journal of Controlled Release. 2004;97(393-405).
- Desai D, Wang J, Wen H, Li X, Timmins P. Formulation design,challenges, and development considerations of fixed dose combination (FDC) of oral solid dosage forms. Pharmaceutical Development and Technology. 2013;18(6):1265-76.
- Mandal U, Pal TK. Formulation and in-vitro studies of a fixed-dose combination of a bilayer matrix tablet containing metformin HCl as sustained release and glipizide as immediate release. Drug Development and Industrial Pharmacy. 2008;34:305-13.
- Benkerrour L, Galley O, Quinet F, Abebe A, Timmins P, inventors; Multilayered tablet containing pravastatin and aspirin and method, 2004.
- Podezcek F. Theoretical and experimental investigations into the delamination tendencies of bilayer tablets. International Journal of Pharmaceutics. 2011;408:102-12.
- Anuar MS, Briscoe BJ. Interfacial elastic relaxation during the ejection of bi-layered tablets. International Journal of Pharmaceutics. 2010;387:42-7.
- Michrafy A, Diarra H, Dodds JA. Compaction behavior of binary mixtures. Powder Technology. 2009;190:146-51.
- Ullah I, Wang J, Chang SY, Wiley GJ, Jain NB, Kiang S. Moisture-Activated Dry Granulation-Part 1:A guide to excipient and equipment selection and formulation development. Pharmaceutical Technology. 2009;33(11):62-70.
- Ullah I, Wang J, Chang SY, Guo H, Kiang S, Jain NB. Moisture-Activated Dry Granulation Part II:The effects of formulation ingredients and manufacturing-process variables on granulation quality attributes. Pharmaceutical Technology. 2009;33(12):42-51.
- Sinka IC, Cocks ACF. Modelling die compaction in the pharmaceutical industry. Modelling of powder die compaction2007.
- Kottala N, Abebe A, Sprockel O, Bergum J, Nikfar F, A. C. Evaluation of the performance characteristics of bilayer tablets: Part I. Impact of material properties and process parameters on the strength of bilayer tablets. AAPS PhramSciTech. 2012;13(4):1236-42.
- Agatonovic-Kustrin S, Rades T, Wu V, Saville D, Tucker IG. Determination of polymorphic forms of ranitidine-HCl by DRIFTS and XRPD. J Pharm Biomed Anal, 25(5-6): 741-750, 2001.
- Tong HHY, Shekunov BY, Chan JP, Mok CKF, Hung HCM, Chow AHL. An improved thermoanalytical approach to quantifying trace levels of polymorphic impurity in drug powders. Int J Pharm, 295(1-2): 191- 199, 2005.
- Vippagunta SR, Brittain HG, Grant DJW. Crystalline solids. Adv Drug Deliv Rev, 48(1): 3-26, 2001.
- Chawla G, Gupta P, Thilagavathi R, Chakraborti AK, Bansal AK. Characterization of solid-state forms of celecoxib. Eur J Pharm Sci, 20(3): 305-317, 2003.
- Stephenson GA, Forbes RA, Reutzel-Edens SM. Characterization of the solid state: quantitative issues. Adv Drug Deliv Rev, 48(1): 67-90, 2001.
- ICH Harmonised Tripartite Guideline. Specifications: Test procedures and acceptance criteria for new drug substance and new drug products: Chemical substances Q6A. Step 5 version. 65(251): 83041-63, 2000.
- Zhang GGZ, Law D, Schmitt EA, Qiu Y. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv Drug Deliv Rev, 56(3): 371-390, 2004.
- Shah B, Kakumanu VK, Bansal AK. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J Pharm Sci, 95(8): 1641-1665, 2006.
- Alam S, Patel S, Bansal A. Effect of sample preparation method on quantification of polymorphs using PXRD. Pharm Dev Technol, 15(5): 452-459, 2010.
- Suryanarayan R. Determination of relative amounts of the anhydrous carbamazepine in a mixture by powder x-ray diffarctometry. Pharm Res, 6(12): 1017-1024, 1989.




