ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
N Sozhan1, T Senthilvelan2, T Kaliyappan3, E Vijayakrishna Rapaka4,
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The solar Gel pond is an innovative concept which overcomes many of the shortcomings of the conventional gradient solar pond. The non- convective salt gradient layer is replaced by a transparent polymer gel layer, which floats on a NaCl solution (Sea water) used in the storage zone. The polymer gel (Carbowax) used was found to be insoluble in different concentration of NaCl solution with good transmissivity characteristics. An attempt has been made to analyse the performance of the solar gel pond of 0.25 m2 area at Pondicherry, India (Latitude 11.56o N & Longitudinal 79.53o E). Experimental investigation has been carried out during the month of February, March and May 2004. The maximum storage temperature was found to be 60 oC and temperature difference between the storage layer and gel layer was found to be around 10 oC. The outcome of the experimental investigations has been reported in this paper.
Keywords |
| Solar Gel Pond, Polymer gel, Storage temperature, Solar Energy |
INTRODUCTION |
| Solar energy, if appropriately harnessed, could provide an inexhaustible energy, source that could contribute directly to improving the quality of life on Earth. One of the inherent properties of solar energy is that it is a diffuse resource, and we therefore require a large area to collect a substantial quantity of energy. Most solar technique would therefore require a very large investment of capital and material resources to realize world economy based on renewable energy. If the goal is to be approached it appears necessary to deploy extremely low cost solar collectors, such as solar pond, which combines low cost collection with long term storage for a variety of low temperature thermal applications. The solar pond concept is capturing the imagination of an increasing number of people after its successful exploitation in Israel for power generation. There are not many large collection area devices less expensive than pond containing water, and the possibility of large tracts of otherwise unused land generating substantial quantities of heat and electricity creates a powerful image. If the energy collection by a solar pond can be maintained at a reasonably efficient level without too much effort, then solar pond technology will be one of the mixes of technologies that would help to lead the earth‟s population to a future based on renewable energy. |
II. LITERATURE SURVEY |
| Weinberger [12] found that the solar radiation can effect a considerable temperature rise in Non-convecting ponds of about a meter in depth. The temperature that could be obtained in the storage zone would be around 85 to 100 C. He derived a method to calculate the thermal efficiency of the pond as a collector of solar energy and proposed a mathematical model of solar ponds. Rabl and Nielsen [26] presented a model with a convective zone at the bottom region of the pond, which is obviously more realistic. The performance of the ground storage beneath a solar pond was investigated and also a computer simulation for prediction of the performance was carried out by Akbarzadeh and Ahmadi [1][2]. Use of a finite difference method to solve the heat diffusion equation in solar ponds was first suggested by Hull [15] has also used the method to investigate the validity of the assumptions employed by Weinberger, Rabl and Neilsen. A theoretical study on the influence of different parameters on the thermal characteristics of solar ponds has been carried out by Kooi from which valuable results have been obtained. In his article, a solar pond is treated as a flat plate collector under steady state conditions and consequently, the Hottel-Whillier-Bliss formula which is commonly used for flat-plate collectors has been also employed as the efficiency equation for solar ponds. However, in Kooi‟s analysis, the bottom heat losses are ignored, and application of his results may lead to an over estimation of pond performance. Hull [15] developed a computer model of salt gradient solar pond thermal behaviour and verified the validity of assumption of constant salt solution physical parameters and ambient temperature and insolation in solar pond analytical models. Shah et al. [27] developed a dynamic computer model of a salt gradient solar pond as an annual-cycle solar energy collection and storage system. The results of the simulations were used to establish a relationship between the system performance and the present design and for sizing the solar energy collection and storage system. Kaushik and Bansal [4][5] reported detailed simulations of a salt gradient pond performance involving optimization of different pond parameters such as the rate of heat removal, the thickness of the nonconvective as well as the convective zones. Hawlader and Brinkworth [11] discussed on the thermal behavior of the non-convecting solar pond. They developed numerical solution for the dynamic equations, incorporating detailed representation of the losses from the surface and using the hourly meteorological data for a site in Southern England. They showed that the pond temperatures are strongly dependent on the effective extinction coefficient for solar radiation and the thermal losses from the pond bottom. Wang and Akbarzadeh [30] presented the optimum thickness of the density gradient layer under various conditions and also discussed on the effect of ground losses for wet soil where underground water level is high. Akbarzadeh [3] discussed the effect of sloping walls on the salt concentration profile in solar ponds. He also obtained the general expression for salt concentration as a function of depth and presented the results for different pond configurations and also for different top and bottom salt concentration. Hillel Rubin, Barry A. Benedict and Stefan Bachu, [13] have applied a finite difference implicit model in order to investigate the interaction among physical variables represented by various dimensionless parameters by considering that the convection currents in the pond are inhibited by salinity distribution. Hull et al [14] modeled the ground heat losses for various perimeter insulation strategies and several pond sizes. They concluded that the uninsulated sloping sidewalls are slightly more effective than insulated vertical sidewalls, expect for very small ponds. They also reported on the experimental results for ground heat loss and energy balance in the 400 m2 solar pond at the Ohio State University. Katti and Bansal [22] investigated the performance of 3 zone solar pond with a diffusely reflecting bottom under two modes of heat extraction. They concluded that the efficiency of solar pond decreases with increasing values of bottom reflectivity. Kishore and Joshi [23] developed a steady state model taking into account the heat losses from the surface and bottom of the pond. The efficiency equation thus obtained is independent of the surface zone temperature and depends only on the geo-climatic parameters. Ebtism Wilkins et. al [8] discussed about the inherent problems encountered with the conventional salt gradient pond leading to the concept of the Solar Gel Pond in which the salt gradient is replaced by the transparent gel layer. They discussed about the relevant properties of the gel. Mehta et al. [24] analyzed the performance of a bittern-based solar pond of an area of 1600 m2 located at Bhavnagar, India. The thermal efficiency of the pond was worked out using correlations proposed by Kooi and Hull. Newell et al. [25] reported on the construction and operation activities at the University of Illinois salt gradient solar pond. Tabor and Dorbon [28] reported on the design, construction and operational experiences of a 5 MWe solar pond power plant. Ebtism Wilkins [9] discussed the design, construction and operation of trapezoidal 400 m2 and 5 m deep gel pond. The pond obtained maximum temperature of 60 C with optimal gel thickness of 60 cm. Sherman and Imberger [6] discussed on the control strategies designed to provide successful high temperature operation of a solar pond year round. They tested the Alice Springs solar pond and were able to maintain temperature in excess of 85 C for several months. Zangrando [31] discussed on the hydrodynamic issues like mass and energy balance; formation, stability, and maintenance of the gradient layer; energy extraction from the bottom mixed layer; stability of stratified fluids to shearing flows; interface dynamics; and wall effects that affect the performance of the solar pond as an energy collector and storage. Jaefarzadeh [20], reported on the performance of a laboratory–scale salt gradient solar pond for different methods of saline injection to the bottom layer and the corresponding temperature and concentration profiles as a function of depth has been reviewed and compared with experimental results. M.Hussain et al [16][17][18] proposed two simple empirical formulations for estimating the radiation flux in solar ponds. First Formulation is developed by empirically extending the formulation of Bryant and Colbeck so as to incorporate the effect of multiple reflection, which is originally expressed for incoming radiation flux only. The other formulation is a fourth order polynomial function of x /L cos , where is the incidence angle of the formulation is compared with Hull‟s model. The proposed formulations are found to provide simplicity in analytical calculations which saves the computational time with a satisfactory accuracy. M.Hussain et. al [16][17][18] analyzed the combined effect of the bottom‟s reflectivity and water turbidity on the steady state efficiency of pond. |
III. SOLAR GEL POND |
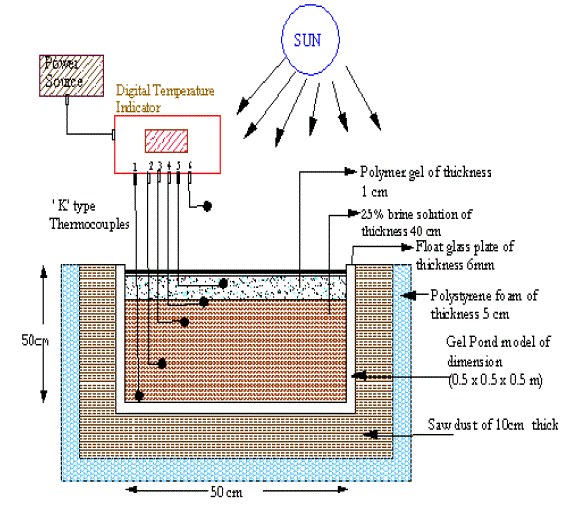
| The concept of Solar Gel Pond is based on the presence of a Non Convective Zone to trap the solar energy. In a salt gradient pond, the variation in density as a function of salinity and temperature gives raises to this NCZ. By contrast, in the Solar Gel Pond the optical and thermal insulating properties of polymer gels are utilized in forming the NCZ. In a solar gel pond as shown in Figure 1, the Gel floats on the storage zone, which acts as the NCZ. At present, 3 to 8 % of salt solution is used in the storage zone to keep the gel layer to float on the top. The gel used in the upper layer comprises of 98 % water and 2 % of the appropriate polymer gel. |
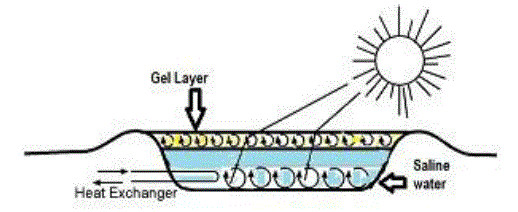 |
| Fig. 1 Schematic diagram of the Solar Gel Pond |
| The dissolved air in the water increases the buoyancy resulting in the floatation of the gel layer. The gel density is lowered due to the addition of dissolved air, which enables the gel to float over 2 % salt water, or even less. Preliminary studies by Wilkins had indicated that there are certain polymer gel which have good optical and thermal properties and could possibly be used in a pond to replace the non-convecting salt gradient zone. During the course of further development of the concept by Wilkins it was decided that to be used as a nonconvecting layer as a transmitter of solar radiation and as a thermal insulator, the gel should satisfy the following criteria: Transparent to visible radiation with very little absorption in all ranges of the solar spectrum. Soluble in cold water before polymerization but insoluble thereafter. High viscosity Chemically and physical stable with respect to a hot saline solution up to 100 C or more Inexpensive High specific heat and low coefficient of volumetric expansion over the operating temperature range of the pond. Inert and nontoxic Non-degradable over repeated freezing melting cycles, and by ultraviolet radiation. Apart from that they are mechanically strong and have stable gel structure. The advantages of the solar gel pond are the elimination of evaporation and heat loss from the surface. The dirt and debris falling into the pond are retained by the surface and can be cleaned off periodically. There are only two zones, lower zone being the saline water and the gel layer floats above the salt water hence no salt gradient layers need be maintained as in the case of solar pond, leading to low maintenance requirements. If an appropriate gel is developed to float on water, then the environment hazard of salt handling can be eliminated. The salt requirements are less in solar gel pond when compared to salt gradient ponds thereby reducing cost and environmental hazard. The disadvantage is the cost of the chemicals required for making the gel is high. |
| A. Modelling of the Gel Pond The Gel Pond has 2 zones namely Upper Gel Layer (UGL) and Lower Convective Storage Zone (LCSZ). Heat loss from the surface of the pond has been accounted. Edge effects due to the absorption of solar radiation as well as heating of side walls are neglected. In this model, the ground heat loss has been considered negligible as assumed by Kaushik and Bansal in their model. Attenuation of solar radiation through the depth of the pond has been calculated. Solar flux at the depth x meters is calculated using the following equation |
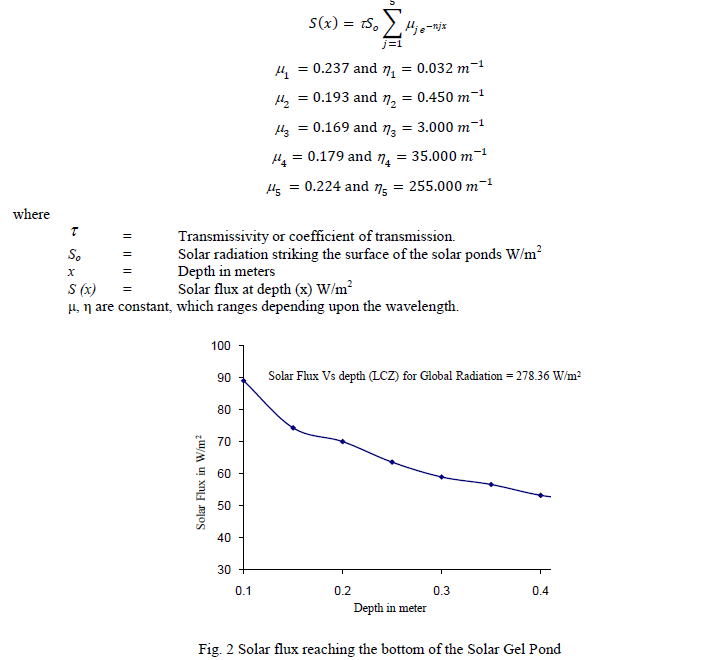 |
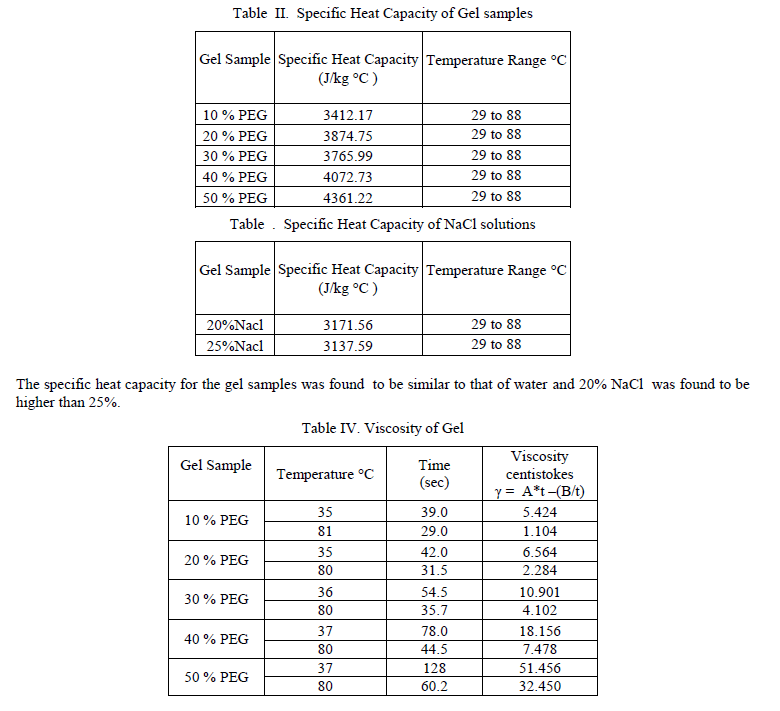
| B. Experimental investigation on a 0.25 m2 solar gel pond Non Convective solar ponds are ordinarily composed of salt gradient layers. Salt gradient solar ponds are found to have a number of difficulties. They cause environment pollution in the event of a salt leakage, and the salt gradient layers needs frequent maintenance. In order to eliminate these faults, a new type of solar pond using a transparent polymer gel as the nonconvecting layer emerged. The polymer gel should have low thermal conductivity which prevents the heat loss through convection and conduction. The polymer gel should not be harmful to the environment. The first experimental study on the gel pond was performed by Wilkins et. al [7] at the University of New Mexico. They reported their results of their experimental and theoretical studies of the gel pond which also included a cost benefit economic analysis comparing the economics of a gel pond with a conventional salt gradient pond. Many thickeners for the nonconvecting layer of the gel pond have been examined and special techniques for the cross-linked polymer gels to form permanent gels have been developed at Battelle Laboratories. Concerning the gel techniques, research and development may still be needed in order to learn how to make a uniform gel on a large scale. Accordingly, at the present status of research, it was recommended that the nonconvecting layer should compose of viscous polymer solution rather than the near state of the solid gel. Other types of salt less solar pond, such as the membrane stratified solar pond which is a body of liquid utilizing closely spaced transparent membranes have been proposed by Hull [15]. However, since the liquids in the nonconvecting layer of this pond are Newtonian fluid such as the sugar solution or ethanol, the membrane space for suppressing convection should be very small and a large number of high transparent films are required. In the present investigation, many natural and synthetic polymers were examined. Carbowax was selected considering the properties like solubility, uniformity, transmissivity for sunlight, low cost, harmlessness, resistance to corrosion and absence of bacterial culture. Outdoor experiments were also carried out for various concentration level of the polymer gel and it was observed that the transmittance decreased due to high scattering for higher levels of concentration. C. Selection of Polymer Gel The work done by Wilkins focused mainly on developing an insoluble and stable polymer gel. A literature survey of possible polymer materials was carried out and the similar procedure as adopted by Wilkins et. al [7] was followed. More than 15 gel materials were identified based on a literature survey and after thorough screening 8 polymer materials were chosen for laboratory tests. Further tests revealed that 5 out of 8 compounds were unsuitable due to the problem of buoyancy and low transmissivity. The following polymers Polyvinyl alcohol, Carbowax were identified as potentially useful and studies were carried out on the above 2 polymers mentioned. The physical properties such as density, specific gravity, viscosity, specific heat and the transmissivity to visible solar radiation for the above polymer gels were measured. D. Evaluation of the properties of Gel. The specific gravity and density has been measured at different temperature for the gel, brine solution and sea water by Hydrometer manufactured by Leimco India – Mumbai. The temperature range chosen was 30 to 88 C as observed from Table 1. Further, it is observed the densities ranges from 984 to 1070 kg/m3 for a typical gel and 1030 to 1198 kg/m3 for different percentage of brine solution at the same temperature. The specific heat was measured by the calorimeter technique. The experimental values of heat capacities are shown in Table 2 and 3 which clearly depicts that the heat capacities of gels are similar to that of water. |
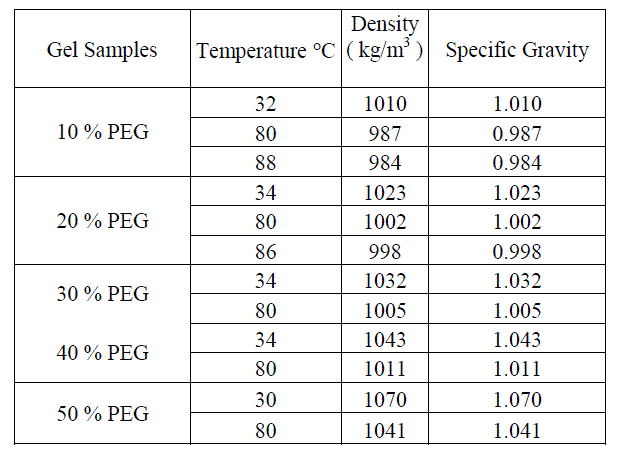 |
| Table I. Density / Specific Gravity of Gel Samples-Hydrometer - Leimco India – Mumbai |
 |
| The gel lifetime is an important factor for its commercial applicability and techno economic feasibility. The following factors Transmissivity, Thermal & UV degradation, Solubility of the gel, Freezing and thawing stability determine the life of the gel. E. Transmissivity Transmissivity of the gel is most important property as far as energy collection is concerned. It was measured with maximum possible accuracy in the laboratory as well as outdoor. Laboratory measurements were conducted using UV Double beam spectrophotometer (Model 2101 Systronics) in the ultraviolet and visible 350 – 800 nm range. |
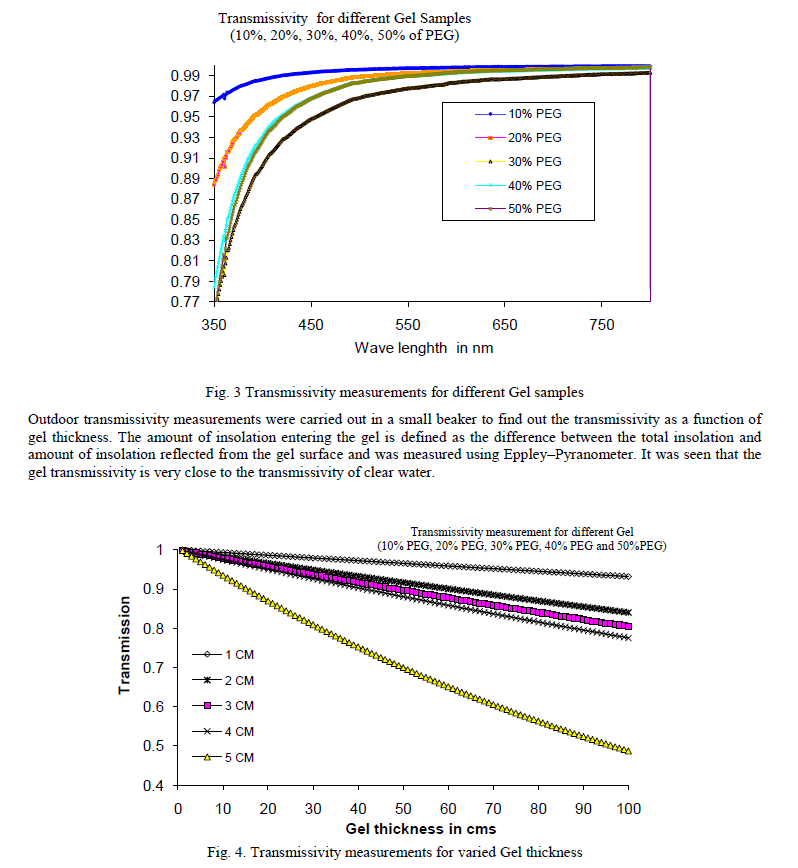 |
| F. Thermal & UV degradation It has been observed that the gel degrades above the temperature of 220 C, above which it starts to break down by evolving gases. Beyond 300 C, a total breakdown of the gel occurs and the gel becomes totally black. G. Solubility of the gel: The solubility of the gel depends on the gelation time which can be varied from 15 minutes to several hours. The gel becomes totally insoluble once the gelation reaction has occurred. H. Freezing and thawing: The freezing and thawing properties of the gel were studied by repeated refrigeration of gel samples at 0 C and then leaving them to thaw outside. Day and night cycles were simulated by leaving the sample in the ambient for 12 hours and then refrigerating them for the next 12 hours. No detectible change was observed in the optical transmission properties of the gel even after 2 weeks of continuous freezing and thawing of the gels. |
 |
| Fig. 5 Schematic diagram of the model Solar Gel Pond. |
IV. DESCRIPTION OF MODEL SOLAR GEL POND |
| Prototype model is square in shape of dimension 0.5 x 0.5 x 0.5 m. Its walls and base were made up of transparent float glass of 6 mm thick pasted with silicon. Inner surface of the glass plate were painted with matt black to absorb solar radiation. The model was insulated with saw dust of thickness 10 cm, polystyrene foam of 5 cm thick to reduce the heat loss through the walls. The top surface of the pond was covered with transparent glass plate of 2 mm plate of dimension 50 x 50 cm in order to reduce the wind effect on the stability of the pond. 6 „K‟ type thermocouples made of Chromel alumel was used to measure the temperatures. Five thermocouples were fixed at 0, 25, 40, 40.5, 41 cm from the bottom and one thermocouple was used to measure the ambient temperature. A 12 channel temperature indicator (MICROSENSOR) was used to measure the temperature of the thermocouples with an accuracy of 1 C. However a thermometer was used to confirm the values indicated by the digital thermometer. Calculated amount of sodium chloride salt was placed on the base of the pond with 2.5 % of total volume added with 100 litres of fresh water. Mixing was carried out in order to ensure that the salt has completely dissolved in the water to obtain a homogeneous mixture. Polymer gel were made by dissolving Carbowax polymer with fresh water. Carbowax was found to be suitable as it satisfied the requirement of the following properties like solubility, uniformity, specific gravity, transmissivity, cost, harmlessness, resistance to corrosion and anti bacterial nature. The pond was filled at the top with polymer gel of thickness 1 cm and the above setup was tested in open atmosphere for one week. In order to study the effect of climate and operational parameters on the performance of solar gel pond, experiment has been carried out during the month of April and May 2004. The global solar radiation incident as a horizontal surface was measured using Eppley- Pyranometer. |
V. RESULTS AND DISCUSSION |
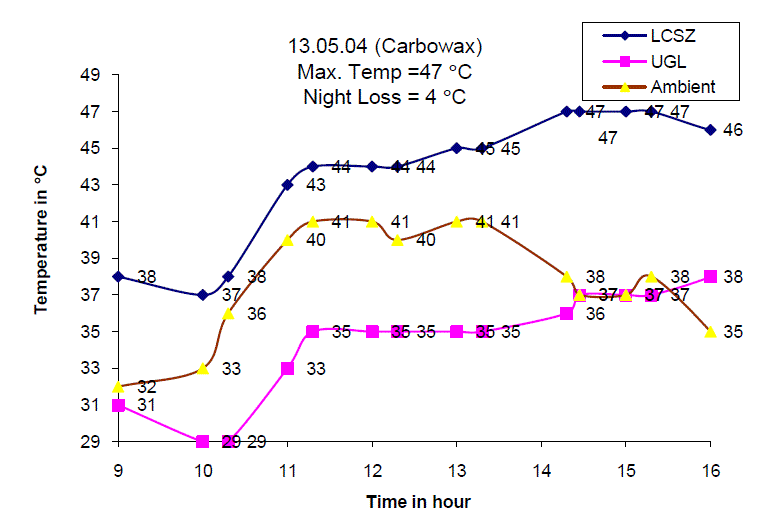 |
| Fig. 6 Temperature distribution of the Solar Gel Pond on 13.05.04 |
| Fig. 6 shows the temperature of the lower convective storage zone, upper gel layer and ambient air temperature on 13th May 2004. It is observed that the maximum temperature of 47 C is obtained around 13.30 hours and the total temperature increase was found to be 8 C. The gel temperature increased from 31C at 9.00 hours to 38 C at 16.00 hours with total temperature increase of 7 C. |
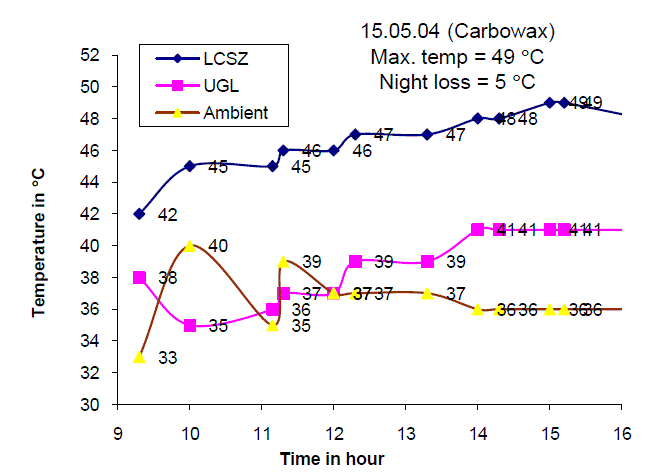 |
| Fig.7. Temperature distribution of the Solar Gel Pond on 15.03.04 |
| Fig. 7 shows the temperature of the lower convective storage zone, upper gel layer and ambient air temperature on 15th May 2004. It is observed that the maximum temperature of 49 C is obtained around 13.30 hours and the total temperature increase was found to be 7 C. The gel temperature increased from 33 C at 9.00 hours to 41 C at 16.00 hours with total temperature increase of 8 C. |
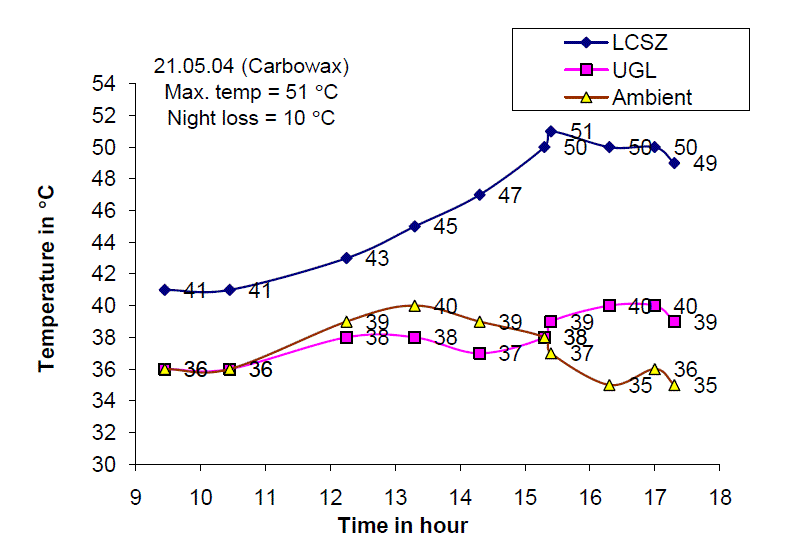 |
| Fig.8. Temperature distribution of the Solar Gel Pond on 21.05.04 |
| Fig. 8 shows the temperature of the lower convective storage zone, upper gel layer and ambient air temperature on 21st May 2004. It is observed that the maximum temperature of 51 C is obtained around 13.30 hours and the total temperature increase was found to be 10 C. The gel temperature increased from 36 C at 9.00 hours to 40 C at 16.00 hours with total temperature increase of 4 C. |
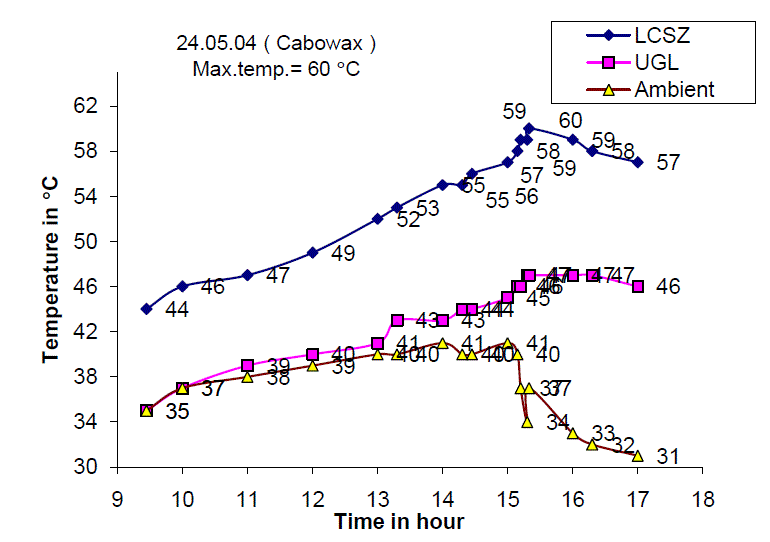 |
| Fig. 9 Temperature distribution of the Solar Gel Pond on 24.05.04 |
| Fig. 9 shows the temperature of the lower convective storage zone, upper gel layer and ambient air temperature on 24th May 2004. It is observed that the maximum temperature of 60 C is obtained around 13.30 hours and the total temperature increase was found to be 16 C. The gel temperature increased from 35 C at 9.00 hours to 46 C at 16.00 hours with total temperature increase of 11 C. |
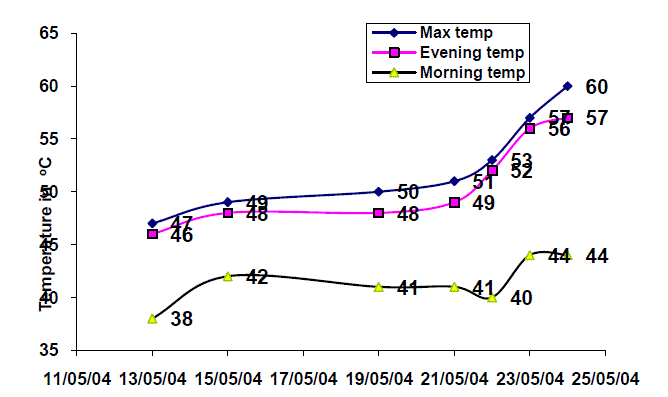 |
| Fig.10. Morning, Evening and Maximum Temperature in the 0.25 m2 Solar Gel Pond |
| The experimental investigations were conducted from 13th May 2004 to 24th May 2004. It was observed that the maximum storage temperature was recorded around 15.30 hours which was found to increase from 47 to 60 C from 13th May 2004 to 24th May 2004 gradually. An increase of 1 C per day was recorded between 13th to 15th May 2004. However the temperature from 16th May 2004 to 18th May 2004 recorded were the same due to cloudy weather. From 19th to 24th May 2004 an increase of 3 C per day was observed. From the graph, it is observed that the gel pond shows an increase in the maximum storage day by day. On 24th May 2004, a maximum storage temperature of 60 C was achieved. From the above experimental investigations, it is concluded that the solar gel pond of 0.25 m2 requires at least 12 days to get stabilized in order to store enough heat to increase the storage temperature of the salt solution to 60 C. It is also observed that the night loss temperature was found to be between 5 to 8 C. The temperature difference between the storage and gel temperature was around 10 C. |
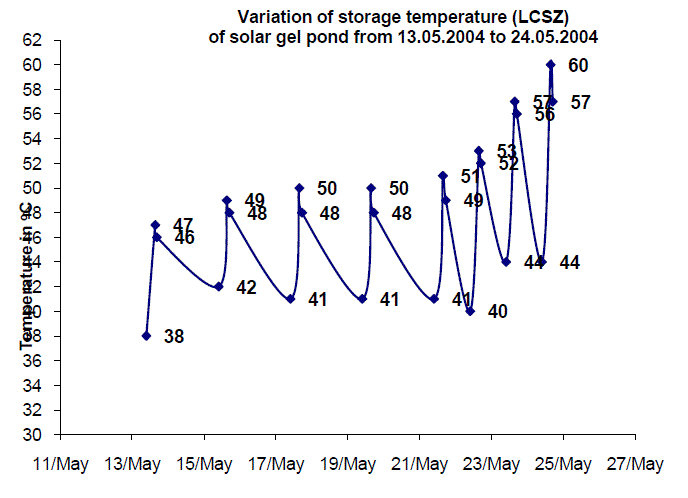 |
| Fig. 11 Performance of 0.25 m2 Solar Gel Pond |
 |
VI. CONCLUSION |
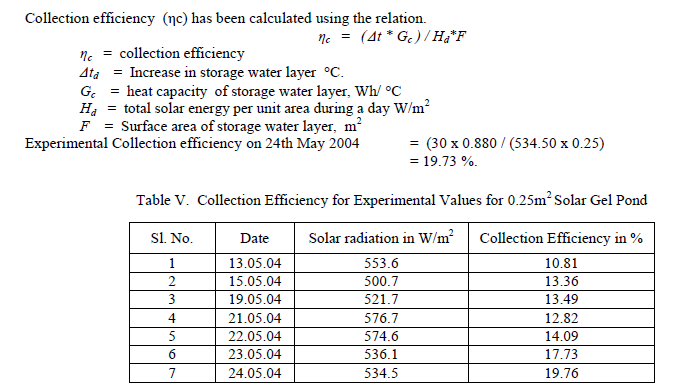
| From the experimental study on the 0.25 m2 solar gel pond, the following conclusions have been arrived. The gel transmittance was found to be around 97.43 % for 1 cm gel thickness. Carbowax polymer was found to be promising as it was found to be stable for longer period without undergoing any reaction with NaCl salt solution. The maximum storage temperature was observed at 15.30 hours which was found to increase from 47 C to 60 C within a span of 10 days. The average temperature difference between storage zone and gel temperature was 10 C Experimental Collection efficiency for 0.25 m2 model for the maximum storage temperature of 60 C is 19.73 %. From the above investigations, the Solar Gel Pond is technically feasible and comparable to the performance of the Salt Gradient Solar Pond. |
References |
|