ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Meena Vangalapati1, Gnana Madhuri Y2, Vidya Sagar T2, Ravi Raju V2 and Satyadev L K2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The radish (Raphanus sativus) species belongs to Brassicaceae family. Different parts of radish including roots, seeds and leaves are used for medicinal purposes. The objective of this work was extraction of phenolic compounds from radish and to develop a modelling equation to describe quantitatively the extraction phenomena. The present study was intended to optimization of extraction of Quercetin and its various physico-chemical parameters have been studied. For the extraction of Quercetin the optimum results were observed for the effect of different solvents, soaking time, extraction t ime with hexane, particle size, different solvent percentages, different volumes of hexane with methanol as solvent and pH where the results showed up are respectively methanol, 1day, 1 hr, 200 particle mesh size, 80% (v/v), 1:1 ratio and 6.0. The highest Quercetin concentration for optimized condition was 45μg/ml. Soxhlet extraction was carried out using methanol for different extraction time periods to verify the mathematical model proposed in this work. The final form of proposed model was Es= 1.525(1-e-0.9033t) for Quercetin, where Es = yield extract (mg of quercetin/gm of dried sample) and t= extraction time (min). The model showed good agreement with the experimental data by generating average absolute relative deviation ( AARD) of about 1.217+- 13.23% mg of quercetin per 1 gm of dried sample
Keywords |
| Radish, Quercetin, Soxhlet extractor, modelling equation, methanol |
INTRODUCTION |
| The radish (Raphanus sativus) species belongs to Brassicaceae family and widely grown all over the world. Raphanus sativus L[1]. is originally from Europe and Asia. Different parts of radish including roots, seeds and leaves are used for medicinal purposes. The most popular part is the napiform tap root, although the entire plant is edible and the aerial parts (stem and leaf) can be used as leaf vegetables. The roots stimulate the appetite and digestion, having a tonic and laxative effect upon the intestines and indirectly stimulating the flow of bile. The juice of the fresh leaves is diuretic and laxative. The seed is carminative, diuretic, expectorant, laxative and stomachic. It is taken internally in the treatment of indigestion, abdominal bloating, wind, acid regurgitation, diarrhoea and bronchitis. The root is antiscorbutic , antispasmodic, astringent, cholagogue, digestive and diureti. It is crushed and used as a poultice for burns, bruises and smelly feet.. This specie is used popularly to treat liver and respiratory illnesses. The main constituents of radish are 4-(methylthio)-3-butenyl isothiocyanate, allyl isothiocayanate, benzyl isothiocyanate and phenethyl isothiocyanate, flavonoids [2] like quercetin and antioxidants. It shows pharmacological activiyies like anti-fungal [3], anti-diabetic [4], anti-oxidant [5], antiinflammatory[ 6] and anti cancer activities [7] etc. |
MATERIALS AND METHODS |
Chemicals and reagents: |
| Methanol, ethanol, ethyl acetate, distilled water, hexane, aluminium chloride, potassium acetate. |
Equipment: |
| Soxhlet extractor, colorimeter, pH meter |
Collection of the plant material: |
| The radish leaves are collected from local market at Visakhapatnam, Andhra Pradesh, India. |
Processing of the plant material: |
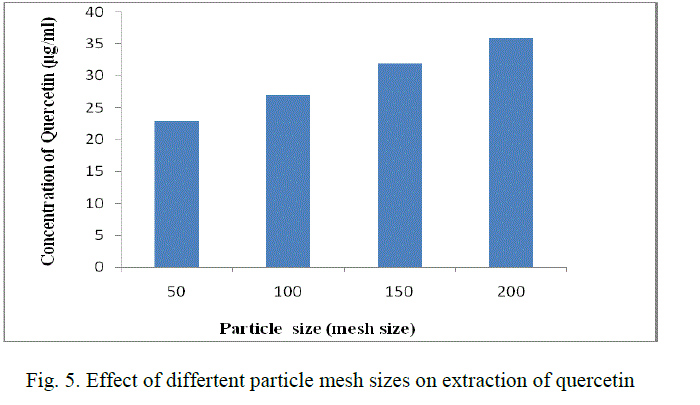
| The leaves are cleaned, dried under shade and powdered. The powder is then screened in different mesh sizes (50,100,150,200) and the different fractions obtained are stored in an air-tight container. |
Extract preparation: |
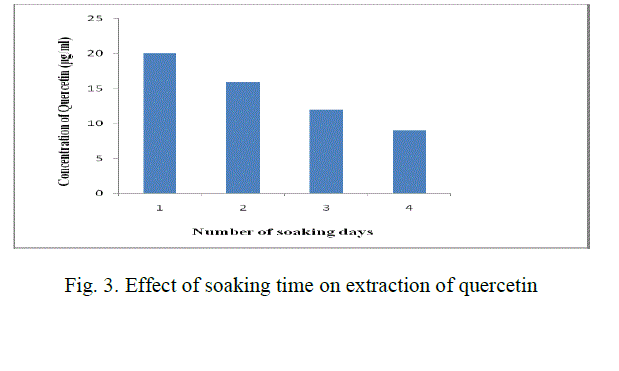
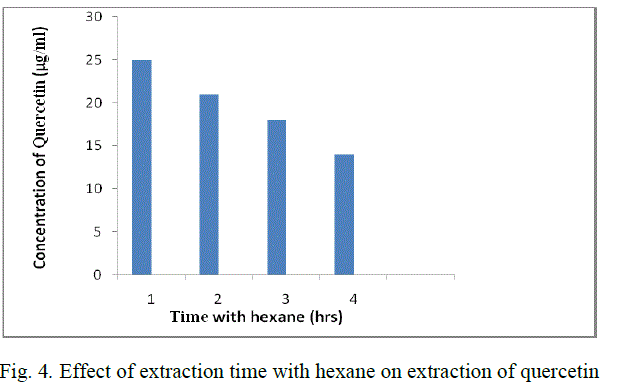
| 2 gm of powder is added to 50 ml methanol (80 %) in a conical flask. The solution is soaked for 1 day. After the soaking time, the solution is filtered using Whatman filter paper no:1 and the filtrate is heated at 65 oC so that the solvent which is taken in glassware is evaporated. The resultant solution is cooled and make up to 25 ml with distilled water. This solution is added to 25 ml of hexane taken in a separating funnel. Incubate the solution of methanolic extract for 1 hr. |
Determination of Quercetin |
| 0.5 ml of methanolic extract is taken in a test-tube from the extract phase of the separating funnel. To this 1.5 ml of methanol, 0.1 ml of 10% AlCl3, 0.1 ml of 1M Potassium acetate and 2.8 ml of distilled are added. This mixture was allowed to stand for 30 min. The absorbance of the reaction mixture was measured at 450 nm using colorimeter. The concentration of Quercetin was determined by using calibration curve. |
Solvent extraction using Soxhlet extractor |
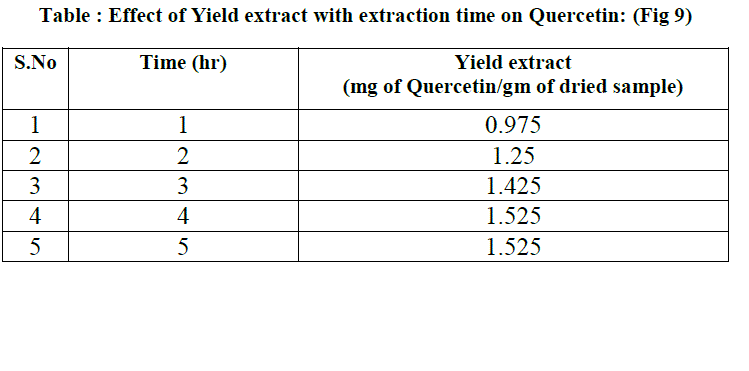
| Prior to the solvent extraction study, 200 ml of 80 % methanol is taken in the extractor. 8 gm of dried radish leaf powder is placed in thimble and it is fixed to the condenser. Now the total apparatus is placed in the heater. Using the soxhlet apparatus continuous extraction is done for 5 hrs where 0.5 ml crude extract is collected for every 1hr for the determination of quercetin. |
Modelling of Extraction of Quercetin using Soxhlet Extractor Apparatus |
| In order to describe the Quercetin transfer from radish leaf powder to the bulk of the solvent the following hypothesis were used. The mass transfer coefficient is constant. The solvent in the extractor is perfectly mixed, while the transfer resistance in the liquid phase is negligible and the Quercetin concentration in the solvent depends only on time. The transfer of the Quercetin was a diffusion phenomenon and independent of time. The final form of the modelling equation[8] was obtained from the extraction of Quercetin by using Soxhlet extractor[9]. |
 |
 |
| A. EFFECT OF PROCESS PARAMETERS: |
| 1. Effect of different solvents on extraction of quercetin : |
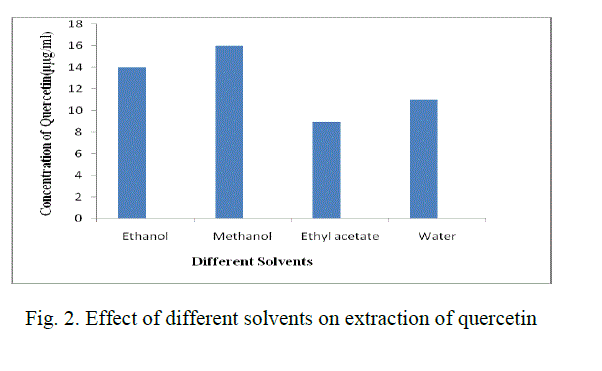
| For the extraction of Quercetin from radish leaf powder, different organic solvents such as methanol, ethanol, water and ethyl acetate are used. Among these, methanol showed the best results and the concentration of quercetin obtained was 16 μg/mL.(Fig. 2) |
 |
 |
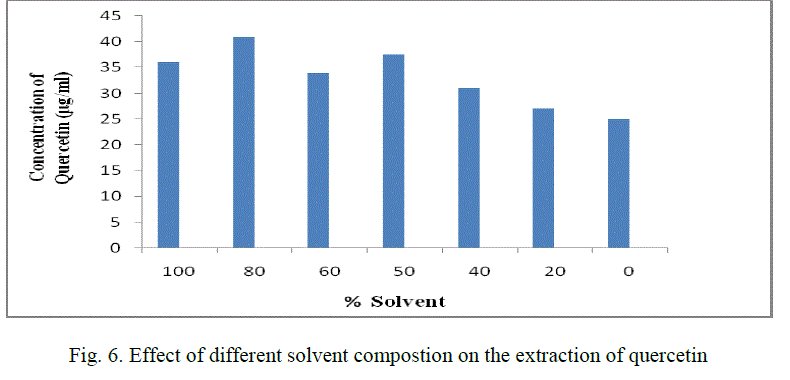
| 5. Effect of different solvent compostion on the extraction of quercetin: |
| Different solvent percentages like 0,20,40,60,80,100 are used for extraction of quercetin. The optimum solvent percentage was found to be 80% and its concentration is found to be 41 μg/ml.(Fig. 6) |
 |
| 7. Effect of ph on extraction of quercetin: |
| To determine the effect of pH on extraction process different pH values namely, 4,5,6,7 and 8 were used. The optimum quercetin concentration of 45 μg/ml was observed at pH 6. (Fig. 8) |
 |
| B. RESULT OF MODELLING OF EXTRACTION OF QUERCETIN USING SOXHLET EXTRACTOR |
| In comparison to non- polar solvents, polar solvents could extract quercetin at higher yield except water, where hydrolysis and thermal degradation might occur. Non-polar solvents were almost unable to extract quercetin. Methanol was found to be the best solvent for the extraction of quercetin. The highest amount of quercetin extracted is from 80% methanol concentration solution. The final form of the proposed model equation for quercetin was |
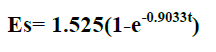 |
| where Es = yield extract (mg of quercetin/gm of dried sample and t= extraction time (min). The model showed good agreement with the experimental data by generating average absolute relative deviation ( AARD) of about 1.217+- 13.23% mg of quercetin per 1 gm of dried sample. |
 |
 |
CONCLUSION |
| The present study was intended for the optimization of extraction of Quercetin and its various physico- chemical parameters have been studied. For the extraction of Quercetin the optimum results were observed for the effect of different solvents, soaking time, extraction time with hexane, particle size, different solvent percentages, different volumes of hexane with methanol as solvent and pH were methanol, 1day, 1 hr, 200 particle mesh size, 80% (v/v), 1:1 ratio and 6.0 respectively. The highest Quercetin concentration for optimized condition was 45μg/ml. |
| Methanol was found to be the best solvent for the extraction of Quercetin from radish by soxhlet extractor. This Solvent extraction using soxhlet extractor was conduct to verify the mathematical model proposed in this work. The final form of proposed models were Es= 1.525(1-e-0.9033t) for Quercetin, where Es = yield extract (mg of quercetin/gm of dried sample and t= extraction time (min). The model showed good agreement with the experimental data by generating average absolute relative deviation ( AARD) of about 1.217 +_ 13.23 % mg of quercetin per 1 gm of dried sample. |
References |
|