ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Anita Sharma1, Vijay Devra2 and Ashu Rani3*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This study investigated the chemical modifications of coal fly ash treated with concentrated HClO4. A solid acid catalyst PAFA was synthesized by the chemical treatment which was prepared from Perchloric acid activation. The physico-chemical modifications of PAFA catalyst was investigated by employing various analytical techniques such as XRF, XRD, FT-IR, SEM and BET surface area. The Brønsted and Lewis acidity of the catalysts were measured by pyridine adsorbed FT-IR. The catalytic performance of PAFA catalyst was evaluated for the series of esterificationt reaction viz. esterification of acetic acid and various alcohols (such as ethanol, propanol, n-butanol, isoamyl alcohol, octanol and benzyl alcohol) to give ester, which are an important fine chemical intermediate, widely used in pharmaceutical and as flavoring agent in confectionary. The catalyst was completely recyclable without significant loss in activity up to 4 reaction cycles, which confers its stability during reaction. The work reports an innovative use of solid waste fly ash as an effective solid acid catalyst.
Keywords |
| Fly ash; mechanical activation; chemical activation; acid treatment and ball milling. |
INTRODUCTION |
| Synthetic chemists continue to explore new methods to carry out chemical transformations. One of these new methods is to run reactions on the surface of solids. Surfaces have properties that are not duplicated in the solution or gas phase; hence entirely new chemistry may occur. Even in the absence of new chemistry, a surface reaction may be more desirable than a solution counterpart, because the reaction is more convenient to run or a higher yield of the product is attained. For these reasons, surface synthetic organic chemistry is a rapidly growing field of study. Esters are essential fine chemicals used widely in industry and agriculture production and daily life [1, 2]. They can be used as pharmaceuticals, flavors, and polymerization monomers, plasticizers in rubber and plastic industry, emulsifiers in the food and cosmetic industry and solvent in chemistry industry due to a low toxicity [3, 4]. Numerous synthetic methods exist for synthesizing various esters. Traditionally, esterification reactions are industrially performed under batch conditions in homogeneous liquid phase by using strong Brønsted acids, such as sulfuric acid, hydrochloric acid or orthophosphoric acid as catalysts [5]. Undoubtedly, these methods are good in terms of reactivity. However, the synthetic scopes of the aforementioned reaction conditions are often hampered by prolonged reaction times, the use of expensive or commercially unavailable materials, high temperature, co-products, strong causticity to equipment, need to be used in quite significant quantities and they are difficult to separate and recover [6] and a great deal of waste |
| water in the process of dealing with product ester and so on. Consequently, methods that successfully minimize their use are the focus of much attention. In recent years, the use of catalysts immobilized on solid supports has received considerable attention. Such catalysts not only simplify the purification process but also help in preventing the release of reaction residues into the environment. Thus, the solvent-free conditions along with the supported catalyst provide a protocol for achieving environmentally friendly economical organic synthesis. For these reasons, efforts to replace the homogeneous catalysts by the heterogeneous ones are being made. Due to their potential benefits, numerous attempts have been made to develop solid acid catalysts to replace liquid acids in chemical industry. Recently, it was continually reported that various kinds of novel solid acid catalysts were used to catalyze esterifications of carboxylic acids and alcohols, such as ion exchange resins [7, 8], solid super acids [9] and heteropoly acids (salts), lipase, sulfonic acids supported on molecular sieves, active carbon or polymers and so on [10-14]. Although these solid catalysts possess high activity and can be easily separated from reaction system, they have also a row of shortcomings: ion exchange resins easily deactivate at high temperature for a long time and show poor reusability. For solid supported catalysts, their application is limited on a large scale because of troubled preparation and high cost. Although Hf (IV) and Zr (IV) salts are highly effective for esterifications of carboxylic acids and alcohols, they are enough expensive compounds. A number of solid acids such as Zeolites [15], resins [16], heteropoly acids [17], and mesoporous silica functionalized with organic acid groups [18-21] and modified zirconia catalyst have also been found to be acidic in nature and catalyze esterification, alkylation and condensation reactions [22, 23]. In our previous work, fly ash has been used for developing several solid acid catalysts by loading cerium triflate, sulphated zirconia and used for the synthesis of aspirin, oil of wintergreen, 3, 4-dimethoxyacetophenone (anti neoplastic) and diphenylmethane [24-27]. Today, there is a significant demand of new and efficient catalyst particularly to design Lewis and Brønsted solid acid together with good catalytic activity, low cost and environmental friendliness. As the thesis is focused on catalytic application of activated fly ash, in the present chapter an innovative catalyst (PAFA) is reported synthesized by chemical activation of mechanically and thermally activated fly ash by concentrated HClO4. PAFA is effectively used for series of organic esterification of different alcohols (ethanol, propanol, n-butanol, octanol, isoamyl alcohol, benzyl alcohol and isobutyl alcohol) with various acids under solvent-free conditions at different temperature. |
II. EXPERIMENTAL METHODS |
| A. Materials and Reagents Class F-type fly ash having (SiO2+Al2O3>80%) produced from combustion of bituminous coal, was collected from Tata Thermal Power Plant (TTPP), Jamshedpur. All chemicals HClO4 (98%), Acetic acid (98%), Ethanol (98%), Propanol (97%), Butanol (99%), Octanol (97%), Benzyl alcohol (98% ), Isoamyl alcohol (98%) and Isobutyl alcohol (99%) were purchased from S. D. fine Chem. Ltd., India and Propanoic acid (99%) from Merck AG. B. Catalyst Synthesis Fly ash (FA) was washed with distilled water followed by drying at 100°C for 24h. Dried fly ash was mechanically activated using high energy planetary ball mill (Retsch PM-100, Germany) in an agate grinding jar using agate balls of 5 mm Φ ball sizes for 15 hours with 250 rpm rotation speed. The ball mill was loaded with ball to powder weight ratio (BPR) of 10:1. MFA was thermally activated at 900°C for 3h to remove C, S and other impurities. The chemical treatment of fly ash was performed in a stirred reactor taking fly ash and 5M aqueous solution of mineral acid (HClO4) in the ratio of 1:2 (FA: Mineral acid) for 5 days at 110°C temperature followed by washing till pH 7.0 with complete removal of soluble ionic species (Cl-, NO3 -, SO4 2-, ClO4 - etc.) and drying at 110°C for 24h. The obtained solid products were calcined at 500°C for 4h similar to our previous work (Scheme 1) [28]. |
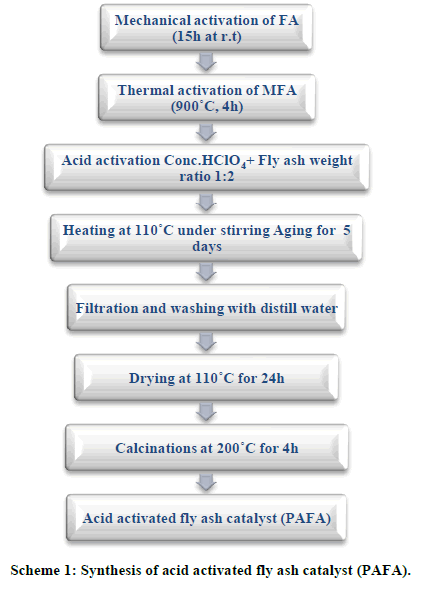 |
| C. Catalyst Characterization The silica content of the fly ash samples after mechano-chemical activations were analyzed by X-ray fluorescence spectrometer (Philips PW1606). The BET surface area was measured by N2 adsorption-desorption isotherm study at liquid nitrogen temperature (77 K) using Quantachrome NOVA s1000e surface area analyzer [29]. Powder X-ray diffraction studies were carried out by using (Philips X’pert) analytical diffractometer with monochromatic CuKα radiation (k = 1.54056 A) in a 2θ range of 0-80o. Crystallite size of the crystalline phase was determined from the peak of maximum intensity (2θ = 26.57) by using Scherrer formula [30] as Eq. (1) with a shape factor (K) of 0.9. Crystallite size = K.λ / W.cosθ…… (1) Where, W=Wb-Ws; Wb is the broadened profile width of experimental sample and Ws is the standard profile width of reference silicon sample. The FT-IR study of the samples was done using FT-IR spectrophotometer (Tensor-27, Bruker, Germany) in DRS (Diffuse Reflectance Spectroscopy) system by mixing the sample with KBr in 1:20 weight ratio [31]. The Bronsted and Lewis acidity of the catalysts were measured by pyridine adsorbed FT-IR (Tensor-27, Bruker, Germany, with DRS. The detailed imaging information about the morphology and surface texture of the sample was provided by SEM-EDAX (Philips XL30 ESEM TMP) [32]. D. Catalytic activity of PAFA The catalytic performance of the nano-crystalline PAFA was evaluated by esterification of acid with various alcohols (ethanol, propanol, butanol, octanol, isoamyl alcohol and benzyl alcohol) to give different esters respectively, as test reactions in a liquid phase batch reactor (Scheme 2). |
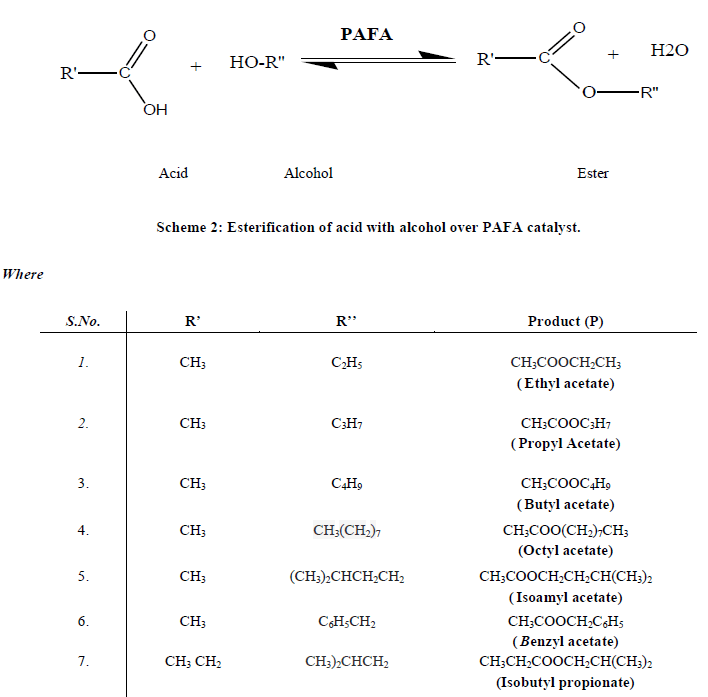 |
| E. Reaction procedure In a typical reaction procedure 3 ml acetic acid and 2 ml alcohol (molar ratio of acid / alcohol = 1.5:1) were taken in a 100 ml round bottom flask, equipped with magnetic stirrer and condenser, immersed in a constant temperature oil bath. The solid acid catalyst (alcohol to catalyst wt. ratio 5), activated at 200oC for 2h prior to the reaction, was added in the reaction mixture. The reaction mixture was refluxed for 1h to 3h at a temperature presented in Table 1. After the completion of reaction, the reaction mixture was cooled and filtered to separate the catalyst. The product obtained was confirmed by melting point measurement, FT-IR Spectroscopy and Gas Chromatography (Dani Master GC) having a flame ionization detector and HP-5 capillary column of 30 m length and 0.25 mm diameter, programmed oven temperature of 50-280°C and N2 (1.5 ml/min) as a carrier gas. The conversion of alcohol was calculated by using weight percent method, the initial theoretical weight percent of alcohol was divided by initial GC peak area percent to get the response factor. Final unreacted weight percent of alcohol remaining in the reaction mixture was calculated by multiplying response factor with the area percentage of the GC peak for alcohol obtained after the reaction. The conversion [33] and yield were calculated as follows: |
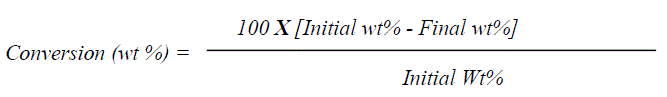 |
 |
| F. Catalyst Regeneration After completion of the reaction, catalyst was filtered and washed thoroughly with acetone and dried in an oven at 110°C for 12h followed by activation at 450oC for 2h in static condition prior to the use in next reaction cycle under the similar reaction conditions. |
III. RESULTS AND DISCUSSION |
| A. Physico-chemical characterization of PAFA catalysts The physico-chemical properties of the fly ash samples before and after chemical activation are given in Table 2 as determined from flame photometry, which shows that silica content in PAFA catalyst is greatly increased from 61.9% to 88%. The increase in silica content after activation shows the loss of significant amount of other components during the chemical activation. The BET surface area of the chemically treated fly ash was greater (26 m2/g) than that of the untreated FA (9 m2/g). The greater BET surface area of PAFA catalyst may be attributed to surface modification of the FA particles by acid solution [34]. |
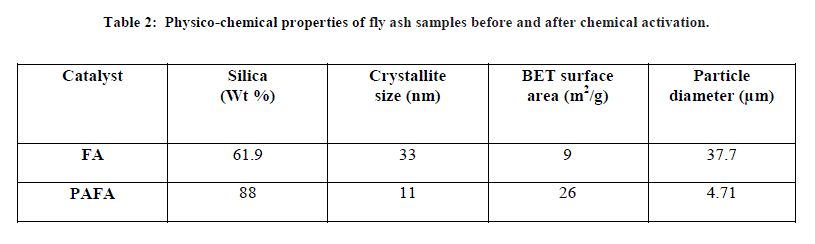 |
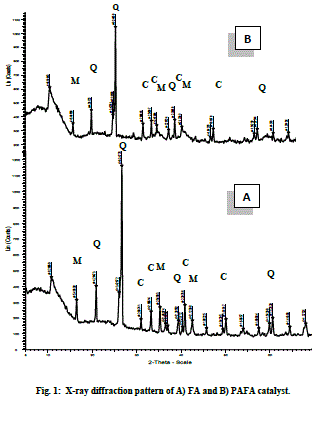
| FA (Fly ash) and PAFA (chemically activated fly ash). B. Structural properties X-ray diffraction pattern of pure FA and PAFA catalyst in Fig. 1A and B shows the presence of crystalline and amorphous phase. Figure also indicates that hexagonal quartz (SiO2) and orthorhombic mullite (3Al2O3•2SiO2) are the main crystallite phases both in FA as well as in the PAFA catalysts. The amorphous phase of FA is increased by chemical activation which results in decreased crystallite size up to 11 nm. Fly ash after chemical treatment with HClO4 is observed to be less crystalline due to formation of silanated silica which is evident from the decrease in relative peak intensities after surface modification (Fig. 1B). The amorphous phase of fly ash is increased more by HClO4 treatment as compared with rest of other three mineral acid (HCl, H2SO4 and HNO3 discussed in earlier chapters ) which results in decreased crystallite size [29]. |
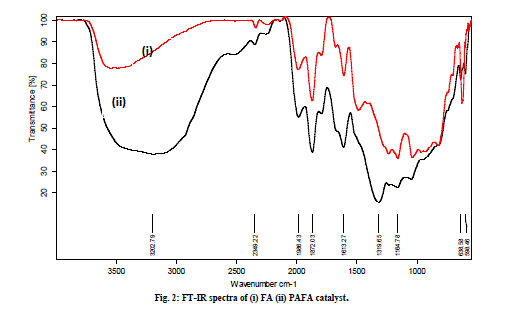
| C. FT-IR studies The FT-IR spectra of FA in Fig. 2 (i) show broad band between 3500-3000 cm-1, which is attributed to surface -OH groups of Si-OH and adsorbed water molecules on the surface. The broadness of the band is due to the strong hydrogen bonding. The hydroxyl groups do not exists in isolation and a high degree of association is experienced as a result of extensive hydrogen bonding with other hydroxyl groups. A peak at 1650 cm-1 in the spectra of both the samples is attributed to bending mode (δO-H) of water molecule. During chemical treatment, the FT-IR spectra of PAFA shows the tremendous increment in broadness at 3500-3000 cm-1 region as compared with FA which reflects strong hydrogen bonding between the hydroxyl groups due to increase C. FT-IR studies The FT-IR spectra of FA in Fig. 2 (i) show broad band between 3500-3000 cm-1, which is attributed to surface -OH groups of Si-OH and adsorbed water molecules on the surface. The broadness of the band is due to the strong hydrogen bonding. The hydroxyl groups do not exists in isolation and a high degree of association is experienced as a result of extensive hydrogen bonding with other hydroxyl groups. A peak at 1650 cm-1 in the spectra of both the samples is attributed to bending mode (δO-H) of water molecule. During chemical treatment, the FT-IR spectra of PAFA shows the tremendous increment in broadness at 3500-3000 cm-1 region as compared with FA which reflects strong hydrogen bonding between the hydroxyl groups due to increase in silica content and loss of significant amount of other components (Fig. 2 (ii). The increased amorphous silica in the activated fly ash can be characterized by an intense band in the range 1000-1300 cm-1, corresponding to the valence vibrations of the silicate oxygen skeleton. The main absorption band of the valence oscillations of the groups Si-O-Si in quartz appears with a main absorption maximum at 1164 cm-1 [26]. |
 |
 |
D. SEM studies |
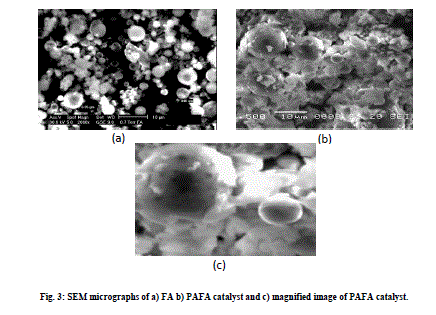
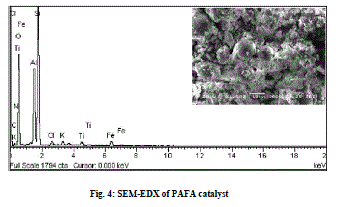
| SEM micrograph of FA indicates that the most of the particles present in the fly ash are spherical in shape with a relatively smooth surface grain consisting of quartz (Fig. 3 a), while after chemical activation with HClO4, silica cenospheres are transformed into agglomerations of large gelatinous mass formed due to the breaking and dissolution of alumino-silicate phases modifying the surface morphology greatly [28] (Fig. 3 b and c). A typical EDX spectrum of the PAFA catalyst is given in Fig. 4, which indicates that the silica content of FA (61.9%) is greatly increased after chemical activation (88%) and content of other metal oxides are found in small proportion. |
 |
 |
E. Catalytic performance of PAFA catalyst |
| In order to understand the effect of activation of fly ash on surface acidity and therefore, the catalytic activity, the catalytic performance of PAFA was evaluated for series of esterification reactions in single step, solvent free condition. |
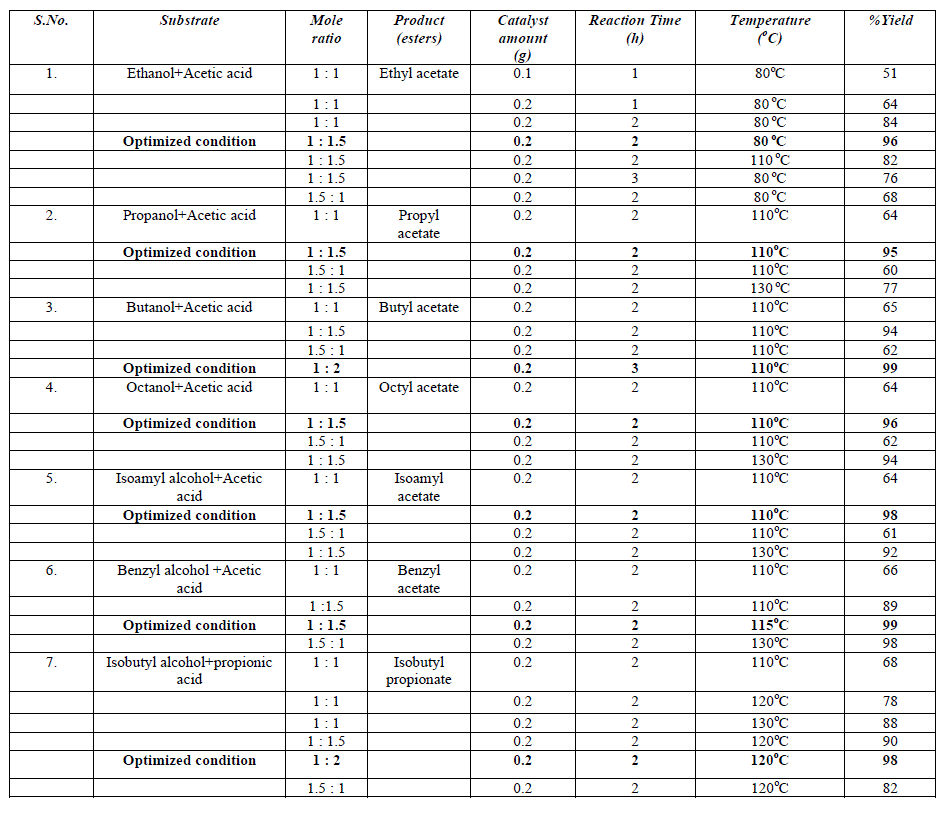
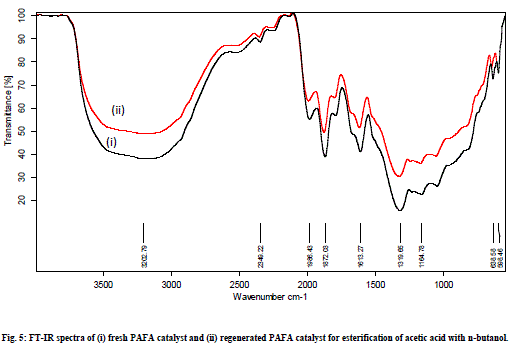
| The esterification of acetic acid with various alcohols was also carried out at different temperature ranging from 80°C to 130°C for 1h to 3h to optimize the reaction temperature, time, and molar ratio gaining maximum conversion of alcohol to esters. Esterification reactions were carried out over pure fly ash (FA) and PAFA by taking varying amount of catalyst, molar ratio of alcohol to acid, reaction time and temperature. The parameters have been optimized and the results of optimization experiments are given in Table 1. It is interesting to note the optimized conditions for maximum conversion are different for different esterifications. However the amount of catalyst remained same. This shows the efficiency of the catalyst for series of esterification reactions. The spent catalyst from the reaction mixture was filtered, washed with acetone and regenerated at 450°C to use for the next reaction cycles. The catalyst was equally efficient up to 4 reaction cycles. It shows that the catalyst is easily regenerated by thermal treatment without loss of catalytic activity (Fig. 5). The conversion was decreased after the 4th cycle, which may due to the deposition of significant amount of carbonaceous material on the external surface of the used catalyst that may block the active sites present on the catalyst. |
 |
F. Proposed Mechanism |
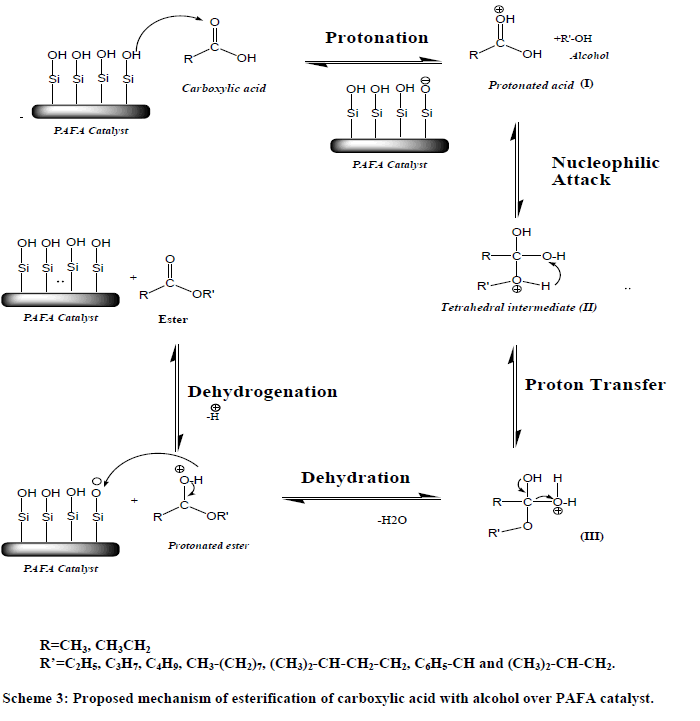
| Esterification take place between acetic acid and absorbed on the acid site of catalyst forming on electrophile and alcohol in the vapor phase, forming ester with subsequent removal of a molecule of H2O. The mechanistic pathways for the esterification of acid are given in Scheme 3, which shows that the catalyst helps in the formation of tetrahedral intermediates providing Brønsted acid sites. |
 |
IV. CONCLUSION |
| The work outlined herein reveals the promising use of PAFA as solid acid catalyst in highly efficient protocol for the synthesis of esters by the esterification of acetic acid with alcohols under solvent-free conditions at low temperature in excellent yields. The remarkable catalytic activity that PAFA exhibited is convincingly superior to other reported catalyst prepared by acid activation with respect to Brønsted acidity. Inexpensive and ready availability of the catalyst makes the procedure an attractive alternative to the existing methods for the synthesis of esters. The catalyst can be called environmentally benign because of its ease of preparation, mildness, and easy work up procedure (filtration). This catalyst can be used and marketed for industrial scale synthesis of esterification organic products for making the process cost effective utilizing the solid waste fly ash in bulk. Many industries have captive power generation with production of fly ash as by product, they can use their own fly ash for synthesizing cost effective catalysts for organic synthesis. |
ACKNOWLEDGEMENT |
| The authors are thankful to Dr. Mukul Gupta, Dr. D.M. Phase, Er. V.K. Ahiray from UGC-DAE CSR Lab, Indore,India for XRD and SEM, SEM-EDX respectively. XRF analysis was conducted at Punjab University, Chandigarh. The financial support was provided by Fly Ash Mission, DST, New Delhi, India. |
References |
|