ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
P. Joe Infant Raj1, M.Uthayakumar2 and K. Sivaprakash3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
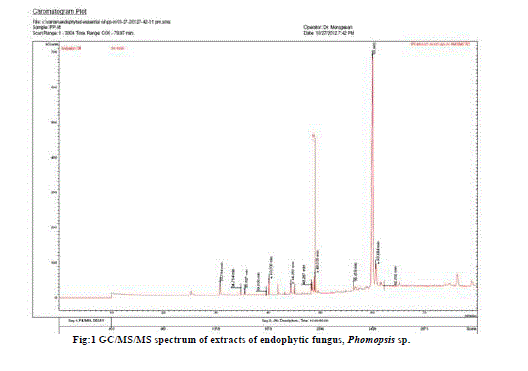
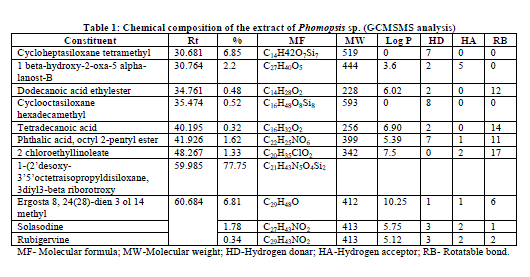
The GC/MS/MS analysis of extracts of an endophytic fungus of entomopathogenic nature, Phomopsis sp. culture filtrate gave us eleven major compounds. All compounds identified by GC/MS/MS screening were assessed for their insecticidal property using physico-chemical property calculations according to Tice Rules. The presence of Dodecanoic acid ethylester, and Phthalic acid, octyl 2-pentyl ester reveled that the extract of Phomopsis sp. might have insecticidal activity. A compund namely, 1-2’desoxy-3’5’octetraisopropyldisiloxane, 3diyl3-beta riborotroxy (C21H43N5O4Si2) is recorded as a major compound with 77.75% which is not reported earlier might have shown promise to use as a source of novel insecticide.
Keywords |
| Endophytic fungus, Phomopsis sp., Tectona grandis and phytochemical compounds, GC/MS/MS analysis, Insecticidal properties. |
INTRODUCTION |
| As ecological damage provoked by extensive use of synthetic insecticides, natural product research continues for the discovery of powerful, selective, and safe alternatives [1]. Many synthetic agricultural agents have been and currently are being targeted for removal from the market, because of profound harmful effects on human health and environment. Thus, perhaps endophytic fungi could serve as a reservoir of untapped biologically based compounds that may present alternative ways to control farm pests and pathogens [2]. Endophytes are microbes that colonize living, internal tissues of plants without causing any immediate negative effects [3]. They reside inside the tissues of nearly all healthy plants. They are synergistic to their host and at least some of them are thought to be useful to the plant by producing special substances, such as secondary metabolites, that prevent the host from being attacked successfully by fungi and pests [4]. One interesting finding consisted in the discovery of peramine, which was toxic to insects without any harmful impact on mammals. This secondary metabolite was characterized in cultures of Neotyphodium coenophialum, N. lolli, Epichloë festucae and E. typhina associated with tall fescue, ryegrass and other grasses [5]. Nodulisporic acids were isolated from a Nodulisporium sp. endophyte in Bontia daphnoides. They were found to exhibit potent insecticidal properties against the larvae of the blowfly [2]. Another endophytic fungus, Muscodor vitigenus isolated from Paullina paullinioides, was found to yield naphthalene as its major product. Heptelidic acid and hydroheptelidic acid, from Phyllosticta sp. an endophytic fungus of Abies balsamea, have been shown to be toxic to spruce bud worm (Choristoneura fumiferana) larvae [6]. Being poorly investigated, endophytes are obviously a rich and reliable source of bioactive and chemically novel compounds with huge medicinal and agricultural potential. Hence, the objective of this study is to identify bioactive compounds in the extracts of endophytic fungus with entomopathogenic significance isolated from T. grandis (teak) leaves using GC-MS technique. This work will help to identify the compounds of insecticidal potential. Three endophytic fungus, Phomopsis sp. was subjected as biological source of the study. Our preliminary studies on the efficacy of this endophytic fungi against teak defoliator, Hyblaea purea gave promising results. This is an eye opening for us to identify the secondary metabolites in the extracts of Phomopsis sp for insecticidal properties. |
MATERIALS AND METHODS |
Collection of plant samples |
| The leaves of T.grandis were collected from Mudumalai Wildlife sanctuary (11° 34´ 37.60Êú N latitude and 76°34´ 05.19ÊúE longitude; 907m MSL), Nilgiris District of Tamilnadu, India. Trees free from insect and disease infestation were selected and marked. Healthy leaves from these healthy trees were collected and processed separately within 48 h of collection. |
Isolation and identification of endophytic fungus |
| The leaves were washed thoroughly in running water and segments of 1 cm2 were cut from the midrib portion of each leaf and surface sterilized by immersing in 70% ethanol for 1 min, followed by 4% sodium hypochlorite (v/v) for 2 min, and finally washed in sterile water for 1min [7]. Each segment from each individual was placed in Petri dishes containing potato dextrose agar (with chloramphenicol 150 mg l–1). Two leaf segments were plated in each Petri dish, the dishes were sealed with parafilm and incubated in an incubator at 26 ± 1°C for 21 days. In addition, 10 ml of the last rinsing water were centrifuged for 10 min at 5000 rpm. The supernatant was removed and added 500 μl sterilized water in the centrifugal tube; 100 μl of this volume were then plated onto PDAS. The surface sterilization was validated because no mycelial growth occurred. The fungi that grew out from the segments were isolated and identified. Pure fungal cultures of the endophytic isolates were obtained by the hyphal tip method in test tube slands. The isolated fungus was numbered and sent to Dr. S. Muthumary, CAS in Botany, Madras University, Chennai (India) for confirmation. Simultaneously, the isolates were stored in 15% (v/v) glycerol at −80°C in deep freezer (VWR Scientific) as spores and mycelium for further study. |
Mass culture and preparation of fungal extracts |
| The endophytic fungus was grown in 2 litre Erlenmeyer flasks containing 500 ml of PDB medium supplemented with soytone [8] and incubated for 21 days. After 3 weeks of still culture at 26 °C, the culture fluid was passed through four layers of cheese cloth to remove solids and extracted with organic solvent. After methylene chloride extraction, the organic phase was collected and the solvent was then removed by evaporation under reduced pressure at 35 °C using rotary vacuum evaporator (Heidolph VV2000) followed by vacuum concentrator (Savant, SC 110). The concentrated extract was subjected to freeze drying in a lyophilizer (Virtis) till dry powder was obtained. Finally the extracted powder was resuspended with methanol at the concentration of 100mg/ml (w/v) followed by filtration through Varian Bond Elute C18 solid phase extraction to remove impurities. 1μl of this solution was employed for GC-MS-MS analysis. |
GC-MS –MS Conditions |
| The GC-MS-MS analysis of the extract was carried out using Varian 4000 Ion trap GC/MS/MS with Fused silica 15m x 0.2 mm ID x 1μm of capillary column. The instrument was set to an initial temperature of 110 ºC, and maintained at this temperature for 2 min. At the end of this period the oven temperature was rose up to 280 ºC, at the rate of an increase of 5 ºC/min, and maintained for 9 min. Injection port temperature was ensured as 250 ºC and Helium flow rate as 1 ml/min. The ionization voltage was 70eV. The samples were injected in split mode as 10:1. Mass spectral scan range was set at 45-450 (m/z). Using computer searches on a NIST Ver.2.1 MS data library and comparing the spectrum obtained through GC-MS-MS compounds present in the extracts were identified.` |
Identification of bioactive compounds: |
| Interpretation on mass-spectrum GC-MS-MS was conducted using the database of National Institute Standard and Technology (NIST) having more 62,000 patterns. The spectrum of the unknown components was compared with the spectrum of known components stored in the NIST library. The name, molecular weight and molecular formula of the extracts samples were ascertained. |
RESULTS AND DISCUSSION |
| The endophytic fungus isolated was confirmed as Phomopsis sp. (Dr. S. Muthumary, CAS in Botany, Madras University, Chennai). The fungus was mass cultured and extracted as the method described above. |
| The GC/MS/MS analysis of extracts of Phomopsis sp. culture filtrate gave us eleven major compounds (Table 1). All compounds identified by GC/MS/MS screening were assessed for their insecticidal property using physico-chemical property calculations according to Tice Rules. As per Tice rule compounds are more likely to have properties of insecticide if molecular weight is within ≥ 150 and ≤ 500; theoretical logarithm of the noctanol/water partition coefficient (log P), is less than or equal to 5.0; hydrogen bond acceptor is within 1-8; hydrogen bond donar is less than or equal to 2 and the number of rotatable bond is less than or equal to 12.. The compounds those are strictly following the Tice rules are considered as anti-insect compound [9]. Two compounds from the extract of endophytic fungus Phomopsis sp. Were observed strictly follow Tice rule. The presence of phytochemicals such as Dodecanoic acid ethylester, Phthalic acid, octyl 2-pentyl ester might be the reason for insecticidal activity of Phomopsis sp. [10,11]. A compund namely, 1-(2’desoxy-3’5’octetraisopropyldisiloxane, 3diyl3-beta riborotroxy (C21H43N5O4Si2) is recorded as a major compound with 77.75% which is not reported earlier might have shown promise to use as a source of insecticide. Hence, detailed study with respect to aforementioned compound will be an insight into develop novel insecticide. |
 |
 |
CONCLUSION |
| India needs an improved and newer group of biopesticides. Biopesticides are only a small part of the insecticide field, but their market is increasing. Biopesticides are biochemical pesticides that are naturally occurring substances that control pests by non-toxic mechanisms. Biopesticides are secondary metabolites that include thousands of alkaloids, terpenoids, phenolics and minor secondary chemicals, derived from plants, microbes, animals, and certain minerals. Several endophytic microbes are known to have anti-insect properties. Endophytes are microbes which colonize living, internal tissues of plants without causing any harm to their host. These endophytes protect their hosts from infectious agents and adverse conditions by secreting bioactive secondary metabolites. Similarly, endophytic fungus, Phomopsis sp. isolated from teak leaves also produced phytochemicals such as Dodecanoic acid ethylester, Phthalic acid, octyl 2- pentyl ester which are reported to have insecticidal activity. Hence, the extract of phomopsis sp. may be considered to use as a novel insecticide. |
References |
|