ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
|
A.Solai, M.Suresh Gandhi, K.Kasilingam and E.Sriraman Department of Geology, University of Madras, Guindy Campus, Chennai -600025, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The Surface sediment samples were analyzed for grain size, carbonates (CaCO3), organic Matter (OM) to obtain a preliminary view of its environmental conditions off Pondicherry along the southeast coast. India. Investigations on the accumulation of heavy metals (Mn, Cr, Cu,Cd, Ni, Pb ,Zn,) in sediments using Atomic Absorption Spectrophotometer at off Pondicherry coast, lying along the southeast coast of India. In sediments, the order was Cr>MN>Pb>Zn>Cu>Ni>Cd. The high concentration of sand in the surface sediments is attributed to the tidal influence at the Virampattinam. The sea water which enters the river suspends the flocculated sediments at the mouth and transports the fine floccules to the water column. The tidal currents also play a major role in removing the fine particles from the river mouth. The value of OM does not show any significant variation towards depth
Keywords |
| Sediment Texture, CaCO3, Organic Matter and Trace Elements |
INTRODUCTION |
| Sediments from the marginal marine and near shore environments had been studied by a number of geologists for a considerable period of time to determine the process of deposition of ancient sediments. The nature of the sediments is modified by anthropogenic activities and as the impact of these activities has increased more in the following years, sediment geochemistry had been pursued with the objective to assess coastal pollution [1-15]. Accumulation or mobilization of trace elements in the sediments of the aquatic environment [16]. Sediments act as sinks and sources of contaminants in aquatic systems because of their varying physical and chemical properties [17-22]. Most of the chemical changes take place in or close to the sediment/water boundary and, for this reason, it is important to study the geochemical composition of bulk sediments [23-24]. Bay of Bengal has attracted scientists and oceanographers from all over the world with regard to its sedimentation geology, in particular, origin and history of the Bengal Fan sediments [25-26]. Sediments are important carriers of trace metals in the hydrological cycle and they effectively collect or release metals into the surrounding waters; thereby, they can reflect the current quality of an aquatic system [27]. Major elemental analysis effectively represents the composition of the solid fraction being eroded from the continents or the rock types present in the drainage basin of a river [28]. Trace metal enrichment in sediments started with the industrial evolution in Indian sub-continent at the end of the 19th century. As a result, the fluxes of trace metals from terrestrial areas (especially aquatic regions) have increased significantly in developing countries. Since, sediments are often the ultimate depository environment for trace metals in the aquatic environment, the solid-phase distribution can reflect the history of metal accumulation and the weathering pattern of the sediments from adjacent regions [29-32]. Normalization is done by measuring the concentrations of metals in bulk sediments and then dividing them by the percentage of 63 μm size fraction in the bulk sediments in order to obtain the concentration in the fine fraction [33-35]. The aim of the present study is to identify the source and fate of metals and toxic elements in the study area, and its impact on the environment. Many reports are available on the physical-chemical features of Indian estuaries [36-46]. Heavy metals in the sediment are essential to assess the extent of metal pollution. |
| The distribution of heavy metals in solution has widely been recognized as a major factor in the geochemical behavior, transport and biological effects of these elements in natural waters [47-51]. Moreover, sediment has aptly been called as „Trace element trap‟ [52] because they eventually receive almost all the heavy metals, which enter the aquatic environment [51]. The scavenging by suspending particles results in a large concentration of pollutants being retained in estuarine sediments [53]. |
| Sediment samples have also been widely used to monitor heavy metal pollution in coastal areas [54,51]. Heavy metal contamination could frequently be identified through analysis. Some studies were carried out on the distribution of heavy metals in water and sediments from Indian regions are limited [55-60, 47-51]. |
| Many investigations have been carried out on adsorption of dissolved metals in estuaries in relation to the role of physical and chemical parameters [61-64]. Heavy metal levels in sediment samples were investigated in various water, Turkish Black Sea, Aegean Sea coasts and Marmara Sea [65-71]. |
STUDY AREA |
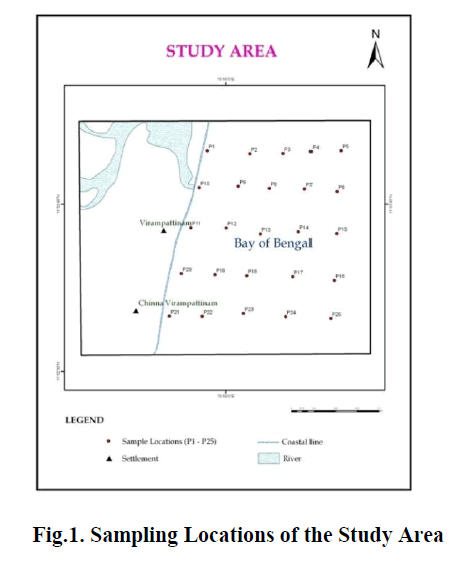
| The present study area extends between off Pondicherry and Chinna Veeranampatnam (Fig.1). It falls in the survey of India toposheets 58m1/16, 58N1/8 prepared in the scale of 1:50,000. The coastal districts have an excellent network of coastal road and railway line connecting Pondicherry to Cuddalore. The study area experiences a tropical sub-humid type of climate with an annual mean temperature of 25oC and average annual precipitation of 1200 mm. |
 |
MATERIALS & METHODS |
| Totally, 25 samples were collected from offshore Pondicherry and Veerampattnam upto 12 mts depth. The offshore samples were collected at grid like pattern at five transects keeping the stations at Off Pondichery, Off Sunambu River, Veerampatnam1, Veerampatnam 2, and Chinna Verampatnam. Global Positioning System (GPS) was used to locate the sample sites in the offshore region. (Fig.2 and Table.1) The coastal sands of recent age overlie the Tertiary rocks. The geomorphologic features observed in this stretch are sub aerial delta, strand plain, crevasses, channels, expat bars, estuarine and swamps. The larger part of the delta is occupied by their distributor flood basins comprising brown and reddish gray silty clay and fine sands. |
| The soils are very deep, moderately drained, clay to sandy clay loam in texture with deposits of sand in intermittent layers. During the first stage of work, sand and mud (silt + clay) were estimated following the procedure of Ingram [72]. Carbonate content (CaCO3) was measured following the procedure of Loring and Rantala [73] and organic Matter (OM) was determined following the procedure of Gaudette et al [74]. Trace elements (Mn, Cr, Cu, Cd, Ni, Pb , Zn,) were determined after preliminary treatment and total decomposition of sediments following the procedure of Loring and Rantala [73]. The final solution was analyzed using AAS (PerkinElmer Analyst 700) in the Department of Earth Sciences, Annamalai University. |
 |
RESULTS AND DISCUSSION |
SEDIMENT TEXTURE |
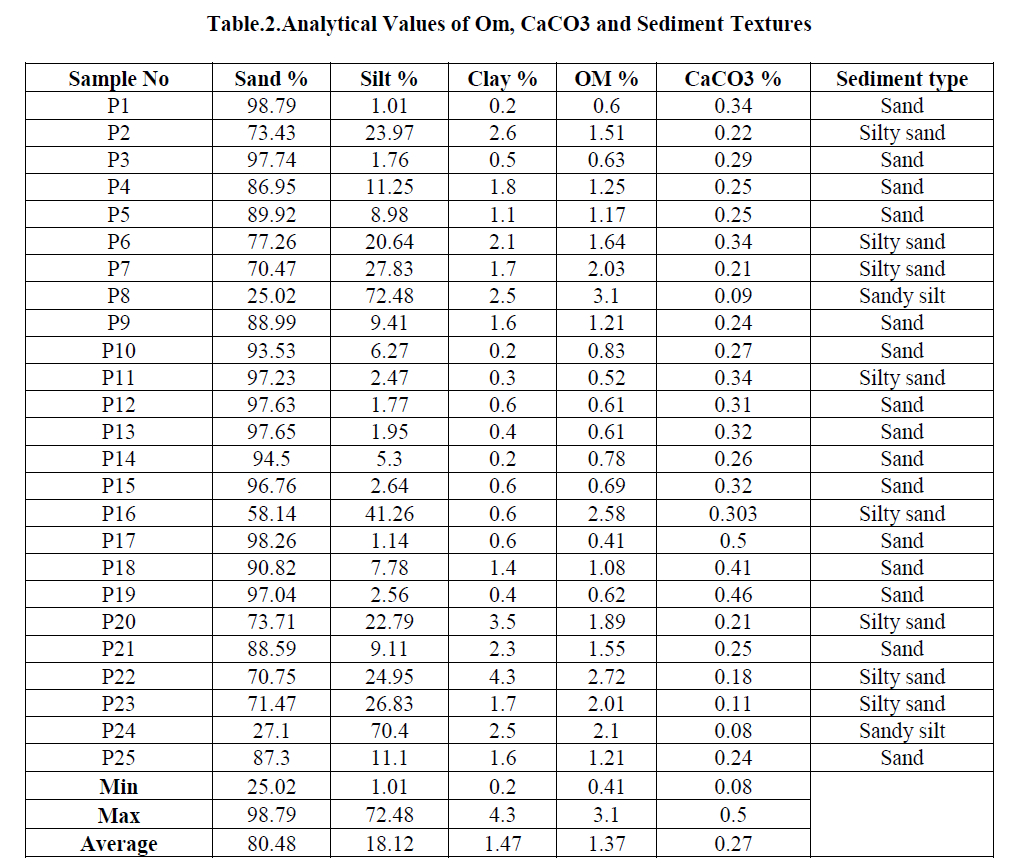
| Grain size parameters have been used commonly to characterize the sediments in the shelf environments [75]. According to Koldijk [76], the analysis of textural parameters is the indicators of environmental conditions. Textural characteristics of the sediment vary with environmental conditions. According to Loring and Rantala [73] the trace metals concentration usually increases with decreasing grain size of the sediments. Hence, the grain size parameters are essential to describe the geochemical characteristics of sediments (Table.2). |
CALCIUM CARBONATE |
| Calcium carbonate is a common substance found as rock in all parts of the world and is the main component of seashells and the shell of snails. Limestones are composed of calcium carbonate and most all are formed from the accumulation of oceanic organisms that make their shells of calcium carbonate. There are shallow water marine limestones and deep water marine limestones. |
| Shallow water limestones are mostly made up of the shell material of reefs, that is corals, bivalves and the like that live in warm shallow waters. In the open ocean, tiny micro-organisms called coccoliths (microscopic plants) and foraminifera (microscopic animals) live in surface waters and they too make their shells of calcium carbonate. According to Emery [77] calcareous sediments are characteristic of low latitudes, and calcareous skeletal sand is widespread at altitudes as high as 60˚. The contrast between the calcium carbonate content of the sediments in intermediate and low latitudes on one hand and in high latitudes on the other is undoubtedly related to the higher production of calcareous forms in low latitudes. |
| In fact, there are virtually no calcareous planktonic forms found in high latitudes. Another factor which may contribute to the difference is that of solution of the calcium carbonate either in the water or after it has reached the bottom. As shown elsewhere, conditions favorable for the solution of calcium carbonate are characteristic of high latitudes in contrast to those in low latitudes. The relative rate of accumulation of noncalcareous material may play some part in determining the general distribution, but it is considered as secondary compared with the absolute rate of supply of calcareous material. |
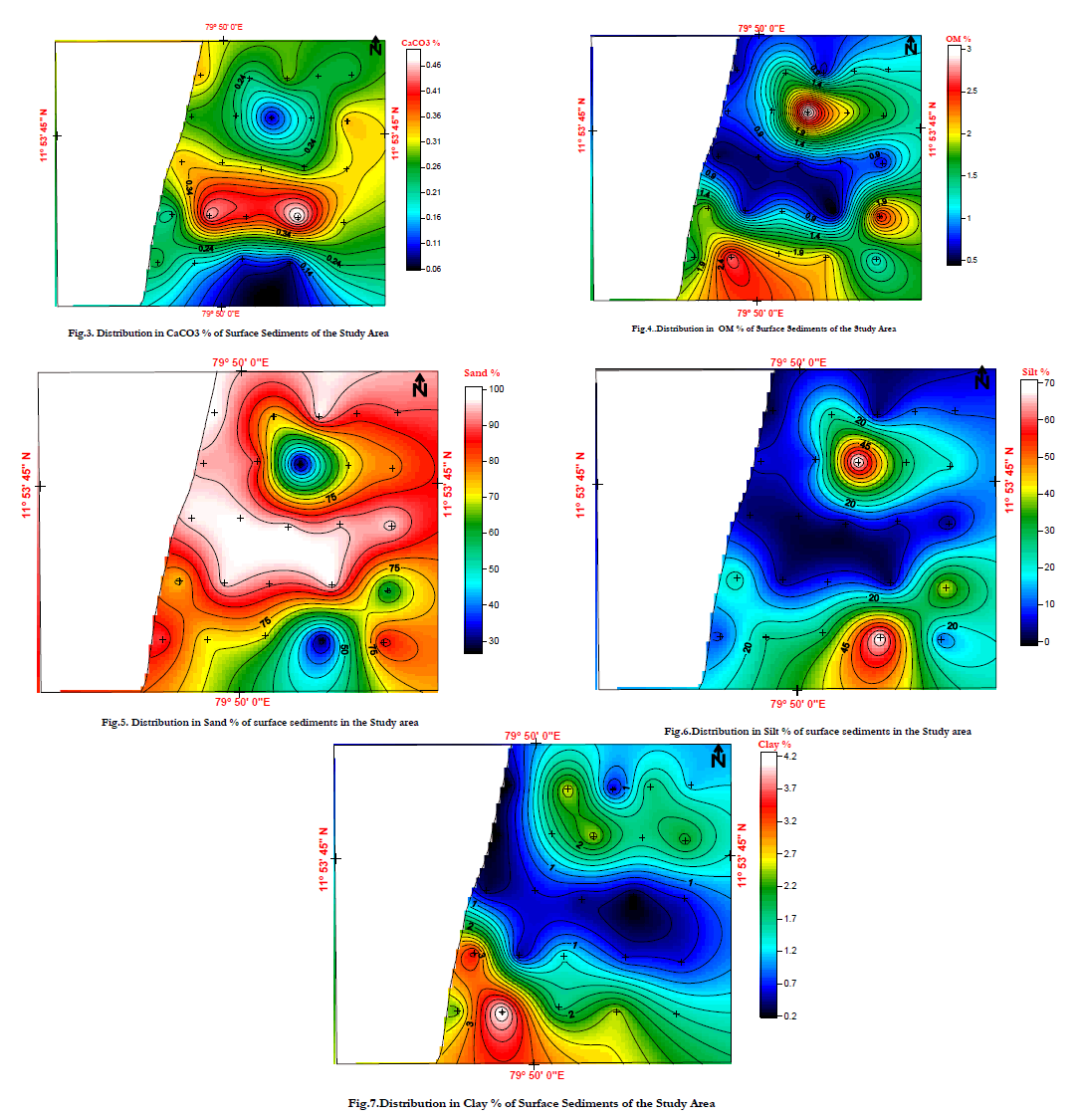
| The lower calcium carbonate content of the sediments may be due to the greater relative rate of accumulation of noncalcareous material or to the slower relative rate of deposition of calcareous material. In the study area Calcium Carbonate contents ranging from 0.08% to 0.5% is noticed. In the nearshore region the Calcium Carbonate is lesser in amount. Veeranampatnam1 & 2 received lower value of organic matter compared to the other stations. In the study area Chunambu River and off Pondicherry receives a higher amount of Carbonate content that the other stations. It indicates that the carbon present in this region is not from in situ. It can be drifted and deposited from the other regions due to northerly littoral drift movement. The decreasing trend of the concentration of calcium indicates dissolution of carbonate due to transient drop in the pore water pH.The principal reason for this may be partial decalcification process [78] due to transient drop in pore water pH. It may also due to biological productivity and its variation through the course of time (Fig.3.) |
ORGANIC MATTER |
| The term organic matter is used to designate that portion of sediment which has arisen through organic activity and which contains carbon in any form other than carbonate. Because it is somewhat analogous to the humus fraction found in soils it may be termed “marine humus.” Sometimes the expression decomposable organic matter is used to indicate that the material may be destroyed by organisms, particularly by bacteria. In the study area organic content ranging from 0.123% to 1.423% is noticed. In the nearshore region the organic matter is lesser in amount. Veeranampatnam1 & 2 received lower value of organic matter compared to the other stations. (Fig.4.) |
SAND-SILT-CLAY RATIO |
| Grain size results indicate a variation of 25.02- 98.79% of sand with an average of 80.48%. Silt ranges from 1.01-72.48% to 18.12% on an average. Clay content in the core sample varies from 0.2-4.3%, with an average of 1.47%. The highest concentration of sand in the core is attributed to the tidal influence at the Sunambu river mouth. The sea water which enters the river suspends the flocculated sediments at the mouth and transports the fine floccules to the water column. The tidal currents also play a major role in removing the fine particles from the river mouth. (Fig.5, 6,7) |
 |
 |
SEDIMENT GEOCHEMISTRY OF TRACE ELEMENTS |
GENERAL |
| Nearshore sediments accumulate in a wide variety of environments are heterogeneous in their textural, chemical and biological characteristics in space and time. These sediments are composed chiefly of terrigenous, calcareous and siliceous materials together with phosphorites and ferromanganese nodules, and cover almost the entire range of sediments accumulating in the oceans at present[87]. Trace elements are those normally present in concentrations less than 1000 μg/g, commonly in the form of oxides, oxyhydroxides, sulphides or organic complexes, and as secondary additives to the detrital mineral matrix. Trace elements include Cd, Cr, Cu, Ni, Pb and Zn (Table.3). |
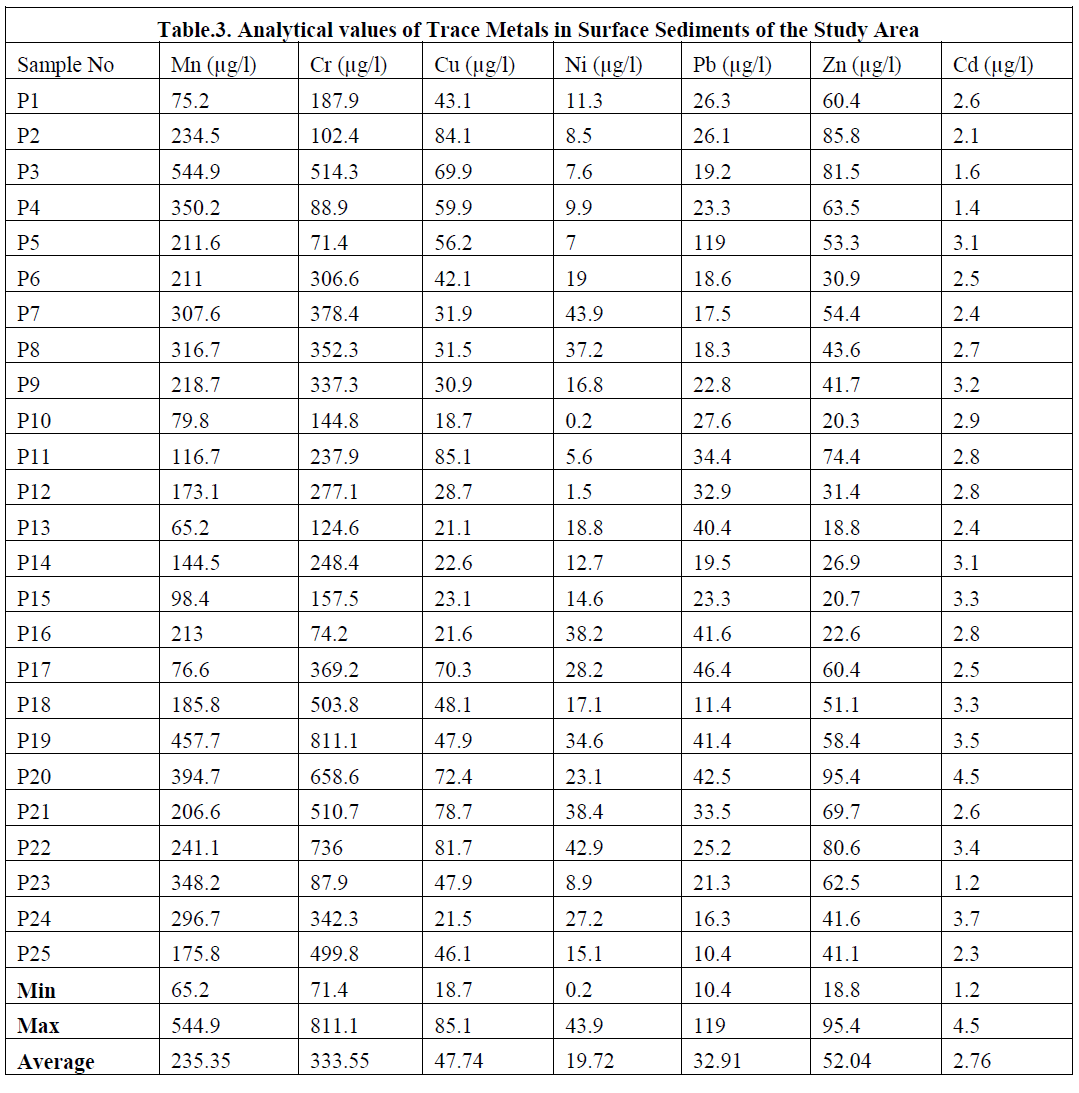 |
MANGANESE |
| The average concentrations of Mn in various geologic source materials such as ultramafic rocks is 1040 ppm; in mafic rocks, it is 1500 ppm, in granitic rocks, it is 390 ppm, in basalts, it is 1500 ppm, in shale, it is 850 ppm, in limestone it is 620 ppm, in sandstone, it is 460 ppm. and in soils it is 320 ppm. The non geologic materials like plant ash have 6700 ppm, in fresh water it is 15 ppb, while the maximum water supply ceiling is 50 ppb. Its lithophile associations are Mg and Fe in silicates and the element is present in most mafic minerals. Manganese exists as Mn oxides in industrial sources and in weathering products. In an acid reducing environment, it occurs as Mn2+. Manganese is an essential nutrient for most plants. |
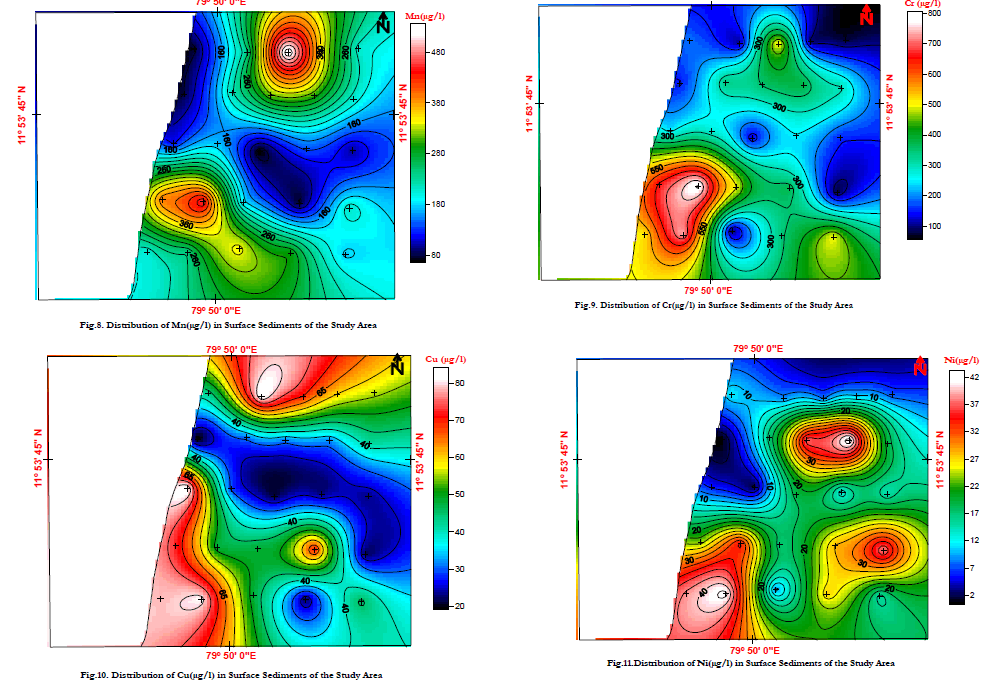
| Its mobility is intermediate to low, except in the acid reducing environment of organic swamps and bogs where Mn can move very readily. In the study area Manganese value ranges from 0.2 – 5.38mg/l, and Average valve is 1.736mg/l. The manganese concentrations of the surface sediments from Bay of Bengal, off Chunnambu river shows decreasing trend in Mn concentration towards the riverine end. At Veeranampatnam and Chinna Veeranmpatnam shows higher concentration. |
| The observed concentrations of Mn are consistent with sediment concentration of Mn in Visakhapatnam shelf where Rao and Sharma [79] inferred the lower values could be due to the sandy nature of the sediments and also due to the low supply of organic matter. The low Mn concentration results due to lack of reducing conditions at depth in the sediment or possible under strong reducing conditions all reactive Mn has escaped to deeper water [80]. The geochemical reactions that occur in estuarine sediments are mostly controlled by the physical chemical conditions in the sediment water complex, in particular the redox potential. (Fig.8) |
CHROMIUM |
| Chromium is one of the least toxic of the trace elements on the basis of its over supply and essentiality. Generally, the mammalian body can tolerate 100-200 times its total body content of Cr without harmful effects. The average concentrations of Cr in ultramafic rocks 2980 ppm, in mafic rocks it is 170 ppm, in granitic rocks it is 4 ppm, in plant ash 6.3 ppm, in fresh water is 1 ppb and maximum water supply ceiling is 50 ppb. When compared with the average value of shale (90 ppm) and Bulk Continental Crustal value (185 ppm) [28] basalts (90 ppm), limestone (11 ppm), soil (70 ppm) and sandstone (35 ppm) which are the main source for marine sediments, have chromium concentration that is much less [81]. Its lithophile associations are Ni and Mg in ultramafic rocks, and it is present in mafic minerals. Cromium3+ can substitute for Mg2+ and Fe3+. However, the distribution of this element in rock forming minerals is further complicated by its ability to form independent Cr minerals such as chromium member of the spinel group, which often occurs in basic rocks [82]. If chromium is present in parent rocks, it occurs along with Fe oxides in normal soils. Its aqueous species are CrO2- 4, Cr2O2- 7. Its mobility is low. In the study area Chromium values range from 71.4 – 811.1mg/l, and Average valve is 333.55mg/l. (Fig.9). |
| The higher concentration of chromium in the marine sediments around the mouth of the Chunnambu River shows that they are the input through the Chunnambu River and could be from the industries on the banks of the estuary. The low concentration in certain samples could be due to the dilution of chromium most probable by high sand content in the study area. |
COPPER |
| Copper is an essential metal in a number of enzymes. Long before copper was recognized as an essential element, it was shown to exist in combination with the blood protein of snails. Excessive intake of copper results in its accumulating in the liver. In most marine sediments copper is present at trace element levels of concentration, well below 100 mg/g. Sources of contamination in natural sediments are often related to mining wastes, industrial metal manufacturing and processing, corrosion products, or as a result of extensive use of antifouling paints in marine areas. Copper is also often associated with sewage sludge, where it is most likely complexes with a variety of organic compounds. It‟s weathering products are sulphides, oxides, basic carbonates, sulphate and silicates over chalcopyrite ore, and Mn-oxides, limonite, organic matter, oxides and carbonates in soils. In the study area Copper values range from 18.7 – 85.1mg/l, and Average valve is 47.74mg/l. (Fig.10). In the Chunambu river region, the concentration is lower. But, in other regions it is higher in concentration. The low input of river flow, the stagnant nature of the water column and anthropogenic input might control the distribution of Cu in the study area. |
NICKEL |
| The overall distribution pattern of Ni in the surface sediments reveals a moderate concentration, which may be due to its incorporation in the dispersed skeletal fragments. Even though the values of Ni show lithogenic origin and low concentration in the present study, they are still rich enough to suggest an anthropogenic contribution. This is confirmed by comparison of the present data with the Adyar estuary [83]. In the study area Nickel values range from 0.2 – 43.9mg/l, and Average valve is 19.72mg/l (Fig.11). |
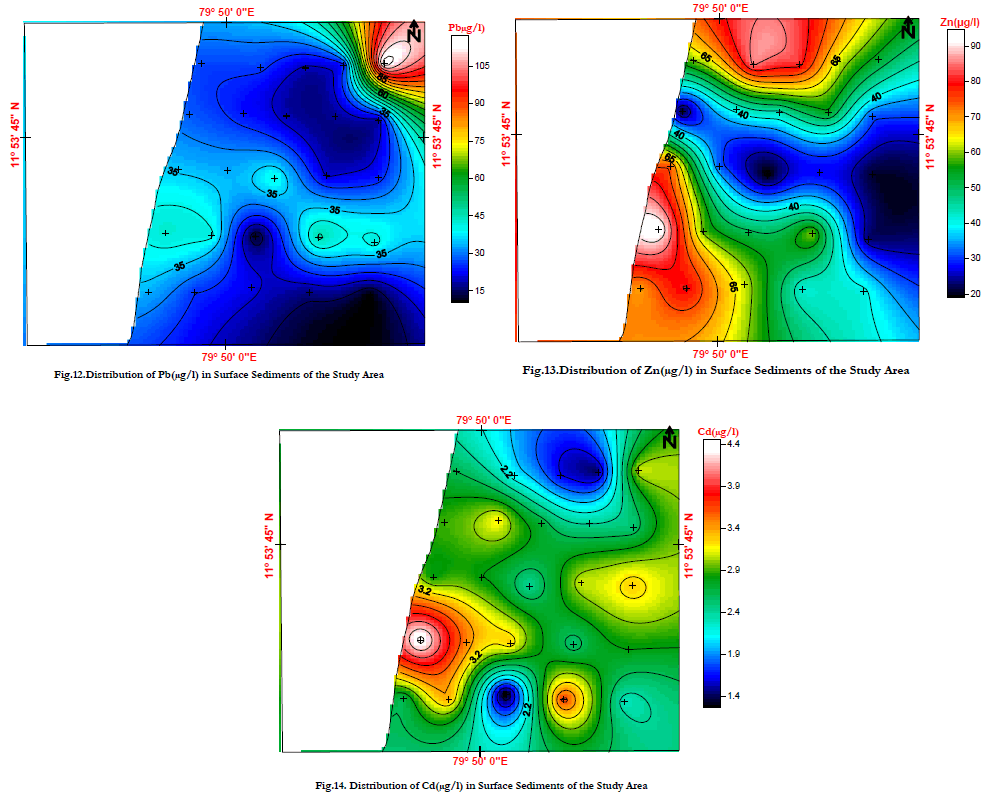
| Lead is a heavy metal that occurs in nature mainly as lead sulphide. This metal is extremely insoluble and is readily adsorbed by organic matter, especially under reducing conditions. According to Buckley and Hargrave [84], the lead sources of environmental contamination are from mining, smelting and reprocessing operations and as a combustion product of lead additives in gasoline. Lead has also been used in a variety of paints and is a common constituent in municipal and industrial wastes. In the study area Lead values range from 10.4 – 119mg/l, and Average valve is 32.91mg/l. The distribution of lead in the surface sediments of the study area reveals a moderate concentration which found well below the reported values of 64mg/g in the bed sediments of river Palar ( (Fig.12). |
ZINC |
| Zinc is an essential trace metal and is an important component of the human body. Its chalcophile associations are Cu, Pb, Ag, Au, Sb, As and Se in base metals and precious metal deposits, and Mg in some silicates. It is present in mafic minerals. Its weathering products are zinc sulphates, carbonates, hydrated silicates, zinc clay minerals over zinc sulphide ores, Fe- and Mn- oxides and organic matter in normal soils. Its aqueous species are variable partition between Zn2+, Zn (OH) 2, soluble organic complexes and floating live organisms. Zinc is an essential nutrient for almost all plants. For this reason, algae growing in streams and lakes can absorb a large part of the zinc dissolved in water. In addition to its nutritive effect, zinc is also toxic to most forms of plants when present in amounts exceeding certain limits. The mobility of Zn is moderately high, limited by its tendency to be adsorbed by MnO2 and by insoluble organic matter. In the study area Zinc values range from 18.8 – 95.4mg/l, and Average valve is 52.04mg/l .The samples collected from Chunambu river shows less content of Zn than the other region. Distribution of Zn concentration expected to control by variations in the grain size of the sediments (Fig.13). |
CADMIUM |
| In the study area Cadmium values range from 1.2 – 4.5mg/l, and Average valve is 2.76mg/l. The Cadmium concentration of the sediments off Veeranampatnam shows higher concentration. From Pondicherry to Chunambu river more or less similar trend is noticed (Fig.14). |
 |
 |
CONCLUSION |
| In between Chinna Veeranampatnam and Pondicherry, a maximum number of strandlines are found to occur around the rivers of Chunnambu river and Uppanar and the same can be attributed to availability of large amounts of sediments to wave action and consequently, for progradation. The grain size data of different environments accounts coarse to Medium to fine sands. From geochemical studies, it is observed that Veeranampatnam and Chinna Veeranampatnam received higher concentrations of trace elements than the other region. Chunnabu river shows not much variation in geochemical studies. |
| The Surface sediment samples are dominated by sand and have a higher concentration of organic Matter and depleted CaCO3 concentration. The highly inconsistent Mn concentration in surface sediments of the present study upholds the idea that oxyhydroxide flocs of Mn are transported laterally during saline bottom water inflow and deposited in quiescent parts of the estuary. When compared to the contamination in other parts of the world, the concentration of trace metals in near shore sediments. High rate of sedimentation and non-decomposition of OM are characterized by the Sediments. |
| The distribution pattern of various trace elements in the Surface sediments is shown as Cr>MN>Pb>Zn>Cu>Ni>Cd Studies on provenance indicate the source for the sediments in the study area are charnockites and tonalitic gneises. Surface sediment samples show a relatively very low degree of alteration in sediments. Their enrichment in the Virampattinam is probably the result of strong summer monsoon currents flowing northward along the coast, and suggests the role of northward along the coast, and suggests the role of coastal currents in redistributing the contaminants that have been added to the estuary by anthropogenic activity. |