ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| J. Marlin Cynthia1*, K. Uma2, R. Gouthami2 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
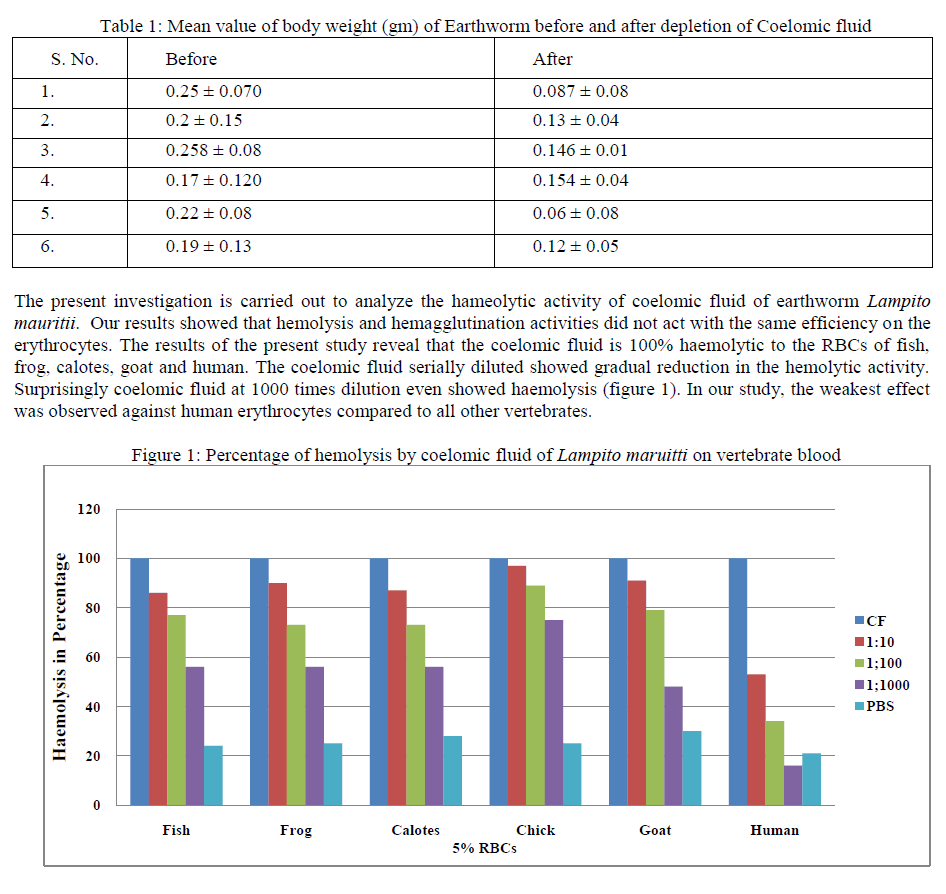
Earthworms have proved to be excellent inexpensive animals for studying regeneration, aspects of neurophysiology, endocrinology and excretion. The body cavity of annelids contains coelomic fluid (CF), in which coelomocytes, the worm’s leukocytes, are suspended. The component of CF of Earthworm defenses them in identifying foreign substance and develops potency to fight against them. The present study is the focused on the lytic effect of CF of Lampito mauritii on the vertebrate blood and sperm would pave the way to trace the immune phylogenetic significance. The Coelomic Fluid (CF) was collected and Hemolytic activity was determined and cytolysis of sperms was observed. The results of the present study reveal that the coelomic fluid is 100% haemolytic to the RBCs of fish, frog, calotes, goat and human. The coelomic fluid serially diluted showed gradual reduction in the hemolytic activity. It is evident that cytolysis and Haemolysis are the properties of coelomic fluid proteins which act as the humoral defense proteins to fight against invading antigenic cells. The coelomic fluid is a fluid system which allows these proteins too efficiently interact with the foreign cells, either bacteria or any vertebrate blood cells and even sperms.
Keywords |
| Earthworm, Lampito mauritii, Coelomic fluid, Hemolytic activity, Cytolysis |
INTRODUCTION |
| Earthworm plays an important role in soil ecology and is the source of food for many animals. Earthworms have proved to be excellent inexpensive, animals for studying regeneration, aspects of neuro-physiology, neuroendocrinology and excretion. Now the attention of comparative immunologists is towards Annelid worms in unweiling the immune protein potentiality in them [1], [2]. The most important characteristics of the immune system of animals in the phylum Annelida are that they have developed a coelom. This body cavity contains coelomic fluid, in which coelomocytes, the worm’s leukocytes, are suspended. Coelomocytes are not contained within the circulatory system but are sensitive to perturbations such as infections and are active in defense reaction ranging from phagocytosis to the more complex mechanism of tissue graft rejection [3]. The component of CF of Earthworm defenses them in identifying foreign substance and develops potency to fight against them. The lysozyme performs lytic activity which can be used to manage the wastes of various organic substances [4]. Moreover, earthworms have been used to treat upper respiratory tract infections, typhoid, and diarrheal pathogenic bacteria as a natural drug in Indonesia more than 50 years [5]. The cytolytic activity of the coelomic fluid was generally demonstrated on vertebrate erythrocytes, and the resulting effect was referred to as haemolysis. The majority of the haemolysis identified so far show haemagglutination activity and, more interestingly a spectrum of antibacterial or bacteriostatic activities against pathogenic soil bacteria [6], [7]. In the cavity of coelom, some easily measurable immune factor like proteases, agglutinins, lysins, and newly synthesized proteins were identified with in protein of CF after suggesting their involvement in immune reaction [8]. Phagocytic phenomenon of coelomocytes releases certain proteinacous lytic substance. The substance can be further explored to move in depth on the hemolytic activity in the vertebrate’s blood samples and in cytolytic activity in the vertebrate mammalian sperms. Therefore the present study is the focused on the lytic effect of CF of Lampito mauritii on the vertebrate blood and sperm would pave the way to trace the immune phylogenetic significance. |
II. MATERIALS AND METHODS |
Earthworms sample collection: |
| The studies were carried out between Novembers 2011 to February2012. Earthworms were collected from Bishop Heber college ground for the present investigation. Collected worms were identified with the key characters described by the scientists of Zoological survey of India (ZSI), and it was confirmed that the collected earthworms were Lampito mauritii, (Kinberg). Earthworms in size range between 15 to18 cm were alone chosen for this investigation. They were weighed and maintained in a container with organic manure. Factors such as pH, temperature and humidity were regularly maintained. |
Coelomic fluid (CF) Isolation: |
| The Coelomic Fluid (CF) was collected as described by [9]. Lampito mauritii, (kinberg) with well-marked Clitella were selected for collection of CF. Earthworms was washed well with distilled water and using what man paper, the worms were wiped clean and wet free.CF was harvested by giving mild electrical stimulation (5V). Fresh CF was used for the study and surplus CF samples were stored in deep freezer. |
Collection of blood sample: |
| All blood samples were collected in EDTA tubes. Blood samples of Chick and Goat was collected from slaughter house. Blood from Fish, Frog, Calotes and Human was drawn in the laboratory. This was centrifuged for 5 minutes at 3000 rpm. The serum fraction was removed and volume was raised to the 2.5ml level with 0.9% NaCl. It was centrifuged for 5 min. The supernatant was again removed and twice to remove the excess serum in the sample. Centrifuged blood is used to prepare 5% RBC solution with Phosphate Buffered Saline (PBS) at required pH 6.8. |
Hemolytic activity:q |
| Hemolytic activity was determined as described by [10], [11]. Freshly collected CF was used for the Hemolytic analysis. Coelomic samples were serially diluted to have concentration of 10-1, 10-2, and 10-3 using PBSCF sample and blood sample preparation were mixed and allowed to incubate at 370C for 3 hours. Blood sample with Distill water is maintained as positive control. PBS alone was taken in a tube to ensure the purity of liquid and used as negative control. After incubation the transmittance capacity was read at 541 nm. From the recorded Optical Density readings, mean values have been calculated and standard deviationfor each sample was worked out. The percentage of Hemolysis was calculated using the formula. |
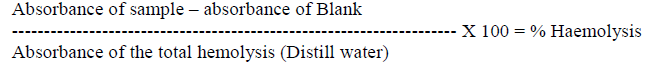 |
Sperm sample preparation: |
| A male frog was dissected and testes were taken out. The testes were teased in 0.9%NaCl and sperm sample was prepared. Testes of Chick were brought from slaughter house and kept in ice. Goat and Buffalo sperms were brought from Tamil Nadu Government Veterinary Polyclinic at Palakkarai. All milky white sperm samples were diluted with WBC diluting fluid and counted in Neubaeur WBC counting chamber. |
Cytolysis of Sperm: |
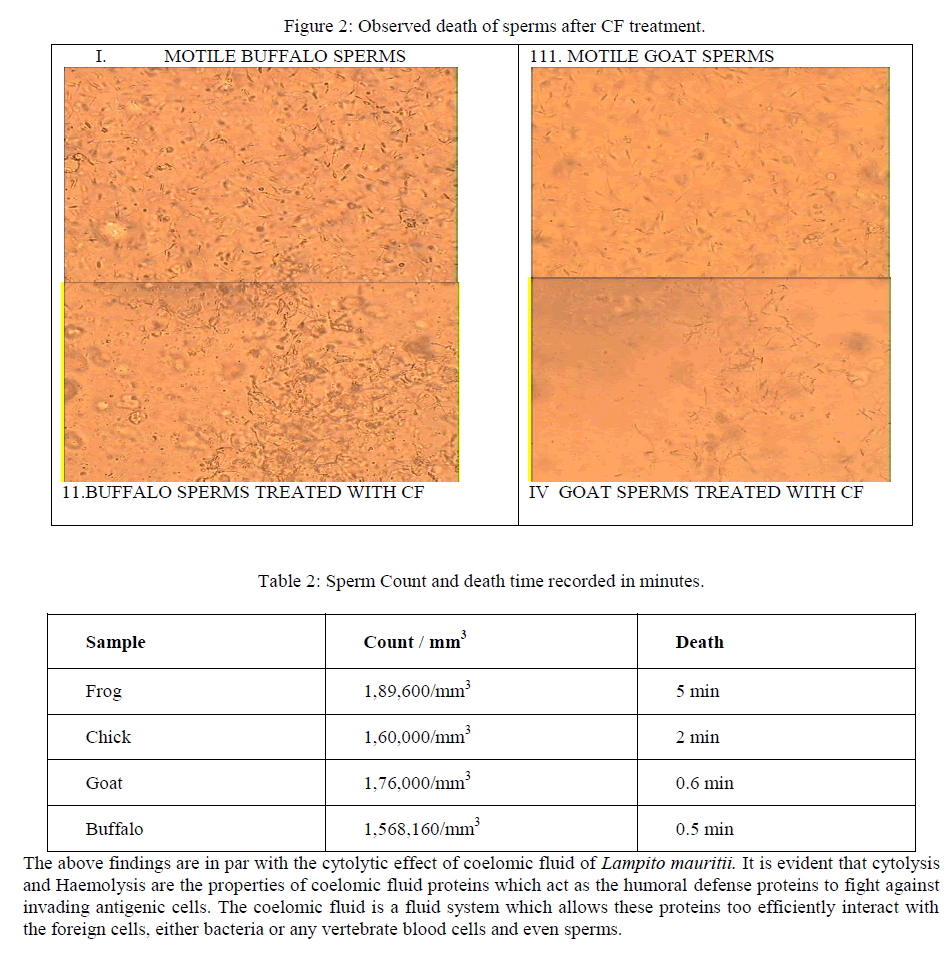
| Sperm death count was estimated as described by [12], [13].Critical observations were made on the motility and the structure of the sample using lower and higher magnifications of optical microscope. Sperm sample is taken in a cavity slide and its motility was photographed. With each sample 0.5ml CF is mixed. The viability and death rate versus time were noted and recorded. The lytic activity and the structure of sperm with saline and CF sample were noted and recorded using photomicrography. |
III. RESULTS AND DISCUSSION |
| In this present study, the haemolytic activity of Megascolidae earthworm, Lampito mauritii has been reported. No such studies, reported so far in the Megascolidae earthworms of Indian Subcontinent. For the first time an attempt is made on the haemolytic ability of coelomic fluid of aneceic earthworm Lampito mauritii. The coelomic fluid of earthworm is known to contain a variety of humoral factors to combat potential pathogens that many migrate from environment into the body [1], [2]. The data Mean ± SD values of body weight of the experimental animal, Lampito maruitii, before and after depletion of the coelomic fluid is shown in the table 1. After depletion of the coelomic fluid the body weight reduced greatly. |
 |
| Kobayashi et al. [14] stated that lysenin, a novel protein that was isolated from the coelomic fluid of the earthworm E. foetida, binds specifically to sphingomyelin (SM) among various Phospholipids found in cell membrane and cause cytolysis. The plasma membrane of mammalian spermatozoa is known to contain SM at relatively high level and therefore the effects of lysenin on the spermatozoa also examined with various animals. Unlike vertebrates invertebrates appear to have little or no SM in any of their tissues [15]. It is likely, therefore, that SM might not be present in on the spermatozoa of invertebrates, lysenine is unlikely to have shows on any lethal effects on the spermatozoa. The sperms of the Frog, Chick, Goat and Buffalo were counted on the Neubaeur WBC counting chamber before being mixed with coelomic fluid. In order to record the cytolytic activity of coelomic fluid 0.5 ml of CF is mixed with 0.5ml of sperm samples and death was recorded (figure 2). The results were, Frog sperm stopped its motility in 5 min, Cock in 2 min, goat in 0.6 min, Buffalo sperm died in 0.5 minutes (table 2). |
 |
IV. CONCLUSION |
| The present investigation utilized the Indian Lampito maruitii in order to evaluate its haemolytic and cytolytic ability. From the findings it was evident that the coelomic fluid is heavily lytic to vertebrate cells. Cytolytic ability of coelomic fluid was carried out on the sperms of buffalo, goat and chick. Lytic effect and death rate of sperms are recorded. Thus the hemolytic and cytolytic phenomenon of CF on the erythrocytes and sperms of vertebrate are much significant findings of the present study. |