ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Subbalakshmi NK 1, Geraldine Monteiro2, Anupama N 3 Sheila R Pai4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Hypertension coexisting with diabetes or isolated is a risk factor for renal dysfunction. But in which of the condition renal function is more affected is not clear. Serum urea and creatinine were measured in five groups: Group A (Gestational diabetics, n = 45); group B (gestational hypertensive diabetics, n = 42); Group C (Hypertension in pregnancy, n = 42); Group 1 (female type 2 diabetics without hypertension, n = 29); Group 2 (female hypertensive type 2 diabetics, n =35). Serum urea was significantly higher in group C compared to group A and group B (p<0.001). Serum creatinine was significantly higher in group C compared to group A (p<0.05). Serum urea was significantly higher in group 2 compared to group 1 (p<0.01). In pregnancy renal dysfunction is mainly associated with hypertension. In female type 2 diabetics renal dysfunction is more severe while hypertension coexisting with diabetes.
Keywords |
| diabetes mellitus, hypertension, pregnancy, renal dysfunction. |
INTRODUCTION |
| Renal failure is one of the leading causes of mortality. Diabetes mellitus is the most common cause of end-stage renal disease and it has been estimated that 366 million people will diabetes mellitus by 2030. [1] Diabetes often coexists with a cluster of other potent cardiovascular risk factors including hypertension. [2] The combined presence of hypertension and diabetes concomitantly accelerates the decrease in renal function. [3] |
| Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. [4] Approximately 7% of all pregnancies are complicated by GDM, resulting in more than 200,000 cases annually.[5] Women with a history of gestational diabetes are at increased risk of cardiovascular and kidney disease, regardless of whether they have gone on to develop overt diabetes, growing evidence suggests. 6 Hypertensive disorders of pregnancy complicate [5–10] precents of all pregnancies and can result in a variety of maternal and foetal complications, including renal failure. [7] In many of the females, hypertension may coexist with gestational diabetes or it may be present separately. Although the hypertension and diabetes mellitus are the known risk factors for kidney failure, which of these clinical conditions aggravate renal function more severely in females is not clear. Therefore this study was undertaken to evaluate the influence of hypertension, gestational diabetes with and without hypertension on renal function in pregnant women. This study also studied the influence of hypertension coexisting with type 2 diabetes on renal function in non-pregnant female subjects. |
MATERIALS AND METHODS |
| This study was done in teaching hospitals attached to Kasturba Medical College after obtaining permission from ethical committee of the institution overseeing human studies and consent from the study participants. |
| 131 consecutive mothers during 20th week of gestational period and 64 female type 2 diabetics in whom fresh medical report on serum urea, serum creatinine and random blood sugar was available were enrolled in to the study. Based on the complications study subjects were divided into subgroups as follows. |
| 131 pregnant mothers were divided into three subgroups: Group A (n = 47): mothers with gestational diabetes but without hypertension; Group B ( n = 42): Mothers with gestational diabetes and hypertension; Group C ( n = 42) : Mothers with hypertension alone |
| 64 female type 2 diabetics were divided into two subgroups: type 2 diabetics without hypertension (group 1, n = 29); type 2 diabetics with hypertension (group 2, n = 35). |
| In all the subjects, height and weight was measured and body mass index was calculated using the formula weight in kilogram divided by height in meters squared. Systolic and diastolic blood pressure was measured in sitting position. Data was analyzed employing unpaired student t test, analysis of one way variance followed by multiple comparisons. When data was not uniformly distributed non-parametric methods namely Kruskal-Wallis test and Mann-Whitney test was used. p value less than 0.05 was taken as statistically significant. |
 |
 |
RESULT |
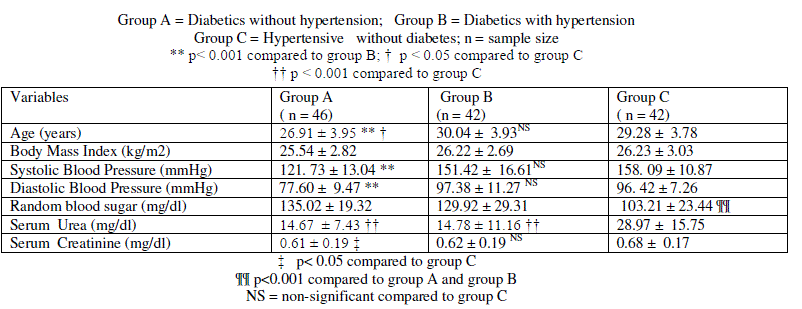
| Data is presented as mean ± SD. Comparison of data different subgroups of pregnant women is presented in table 1 and data on subgroups of female type 2 diabetics is presented in table 2. In pregnant women, mean age of mothers with gestational diabetes alone (group A) was significantly lower compared to mean age of mothers with combined gestational diabetes and hypertension ( p< 0.001 group B, table 1); and compared to hypertension alone group (p < 0.05, group C, table 1). Mean systolic blood pressure and mean diastolic blood pressure of group B and Group C was significantly higher compared to group A ( p< 0.001, table 1). Mean age, mean systolic blood pressure and mean diastolic blood pressure of group B did not differ significantly compared to group C. Random blood sugar was significantly lower in group C compared to group A and group B (p < 0.001). Random blood sugar did not differ significantly between group A and group B. Body mass index did not differ significantly among the subjects in group A, group B and group C (table 1). |
| Mean serum urea level was significantly higher in group C compared to group A ( p< 0.001 and group B (p < 0.001, table 1). Serum urea level was comparable between group A and group B. Mean serum creatinine was significantly higher in group C compared to group A (table 1). Mean serum creatinine did not differ significantly between group B and group C (table 1). Mean serum creatinine was comparable between group A and group B. |
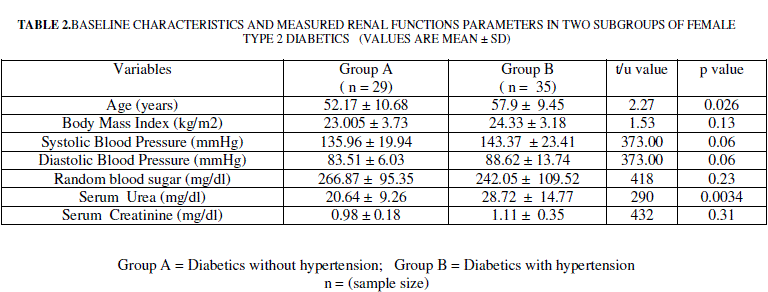
| Female Type 2 diabetics were divided into two subgroups based on the presence /absence of hypertension. Among the 62 female type 2 diabetics included 29 (group 1) were without hypertension (group 1) and 35 were with hypertension (group 2). Data on these two subgroups of female type 2 diabetics is presented in table 2. Mean age of Group 1 was significantly lower compared to Group 2. Group 1 was comparable with regard to body mass index, random blood sugar, systolic blood pressure and diastolic blood pressure of group 2. |
| Mean serum urea was significantly higher in diabetics with hypertension compared to diabetics free from hypertension (p < 0.001, table 2). Serum creatinine did not differ significantly between diabetes alone and diabetes with hypertension groups (table 2) |
DISCUSSION |
| In the present study hypertensive group alone had significantly higher serum urea level compared to gestational hypertensive group (table 1) But the age , body mass index, the systolic and diastolic blood pressure of gestational diabetics with hypertension and hypertension alone group did not vary significantly (table 1). The relation between pregnancy-induced hypertension and gestational diabetes is not well understood. [ 8-13] Several studies suggest an association between these diseases, [8-10] but others do not. [11, 12]However, our finding suggests that the pathogenesis of pregnancy induced hypertension is more severe in impairing renal function compared to hypertension coexisting with diabetes mellitus. Pregnancy induced hypertension, an idiopathic multi-system disorder of the mother, often exhibits a spectrum of pathophysiological changes. More recently, numerous studies have been performed, elucidating the role of platelets in the pathogenesis of hypertension. Changes in the coagulation system coupled with an array of hematological abnormalities, in an established case of pregnancy induced hypertension have been well documented. [14] However further studies are required on pregnancy induced hypertension in understanding the pathogenesis and effective management of it. |
| In the present study serum urea was significantly higher in female type 2 diabetics with hypertension compared to diabetics free from hypertension. Thus our finding supports the view that the combined presence of hypertension and diabetes concomitantly accelerates the decrease in renal function. Therefore goal should be directed in controlling hypertension apart from regulating blood sugar in type 2 diabetics. |
CONCLUSION |
| Based on our study findings it could be concluded that hypertension coexisting with type 2diabetes mellitus impairs kidney function mainly in non-pregnant females. In pregnancy, mothers with pregnancy induced hypertension are at greater risk of renal function impairment than hypertension associated with gestational diabetes mellitus. |
References |
|