ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
C. Rajthilak1, P. Santhanam2, J. Sivakumar3, C.Premkumar3, P.Perumal4*
|
| Corresponding Author: P.Perumal, |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
An investigation was made on the utilization of wastewater collected from the aquaculture farm near Mimisal, Pudukkottai District, Tamil Nadu (South East coast of India) for the culture of harpacticoid copepod Nitokra affinis under laboratory condition. During the culture period, the water quality parameters and microbial populations were monitored before and after the inclusion of copepods in aquaculture wastewater. The culture system with initial stock of Nitokra affinis produced an average of 650 ind/lit (i.e., nauplii- 350, copepodites - 200 and adults - 100) during the 21days of culture period. Further, there was a significant difference found in the concentration of heavy metals and nutrients. There was a gradual decrease found in the population of actinomycetes viz. 3.2×10-3 to 3.0×10-3CFU/ml and pathogenic bacteria (Vibrio) 5.9x10-3 to 3.9x10-3 CFU/ml was noticed. The total heterotrophic bacteria (beneficial microbes) level (CFU/ml) increased to 5.30×10-4from the initial level of 2.12×10-4. The present study suggested that probiotic microbes enhanced the population growth performance of Nitokra affinis in aquaculture wastewater
Keywords |
| harpacticoid, copepod, Nitokra affinis, actinomycetes, probiotic microbes |
I. INTRODUCTION |
| Aquaculture, also known as aquafarming, is the farming of aquatic organisms such as fish, crustaceans, molluscan and aquatic plants. It is the fastest growing food production sector in the world. Fluctuation in abiotic and biotic factors influences the growth and distribution of zooplankton [4]. Wastewater discharged from aquaculture contains nitrogenous compounds (ammonia, nitrate and nitrite) which cause harmful effect to the organism at high concentrations [10].Therefore, nutrient removal is essential for wastewater treatment to protect receiving waters from eutrophication and for the potential reuse of the treated water [11]. Aquaculture wastes comprises of various excrete, wastes, etc which could be decomposed by beneficial microbes and invertebrate detritivores. Recent times, zooplankton (class: Crustacean, phylum: Arthropoda) are used for the assessment of water quality including pollution stress of aquatic ecosystem [2]. In aquaculture industry, live prey selection is mainly based on their biochemical quality. An alternative method of nutrient enrichment of live food organisms is through addition of probiotics. The probiotics plays a significant role in controlling pathogens, stimulating host immunity supplying of vitamins, aminoacids, fatty acides and enzymes which will stay active in host intestine [3],[15], [16],[5],[6],[23]. Recently Sun et al. [19] reported in the copepod Pseudodiaptomus annandalei can function as a suitable probiotic vector for the larvae Epinepheluscoioides. However, no more information is available about the application of probiotics through copepod as a vector. The present study was aimed to culture Nitokra affinis successfully in the existence of probiotic (beneficial) microbes in the aquaculture wastewater. |
II. MATERIALS AND METHODS |
| A. Copepod Culture |
| Zooplankton samples were collected during early morning of full-moon time, using 158 μm plankton mesh (0.35m mouth diameter) from the Vellar estuary (La t .11 °29 ' N and Long 79 ° 46 ' E). The samples were immediately transported to laboratory and vigorously aerated using aerator and thoroughly rinsed to reduce contamination of other zooplankter. Then, the sample was screened to isolate the size fractions containing predominantly adult and later-stage copepodites. This was achieved by a first crude screening through a 500-μm mesh to eliminate the fish and prawn larvae. Then the samples were rinsed for 2hr in a zooplankton washer fitted with a 190-μm mesh screen to remove rotifers, nauplii of copepods and barnacles. After rinsing, the remaining adult copepods and larger copepodites were used to start the culture. A stock culture of Nitokra affinis was maintained in a rectangular, flat-bottomed fiberglass tank (550 mm dia., 850 mm height) filled with 100 litres - filtered seawater with vigorous aeration. Rearing containers were covered with nylon cloth to prevent excessive evaporation. Seawater was filtered through a membrane filter (pore size 100μm). Contamination of the rearing tank was reduced by daily water changes. Temperature (°C), salinity (ppt) and pH were maintained in the ranges of: 28 - 32, 30 – 34and, 7 - 8.5 respectively. During culture, the copepods were fed with equal quantities of Isochrysis galbana, Nannochloropsis salina and Chlorella marina in the ratio of 3:3:3 (30,000: 30,000: 30,000 cells/ ml of each algal species). The stock cultures were harvested every 13th day by gentle siphoning and the components of the culture tank were transferred to a zooplankton washer and then rinsed for 1h with seawater (from the reservoir). Adult s and copepodites collected from the zooplankton washer were used to restart the stock culture. The adult female gravid copepods (Nitokra affinis) were picked carefully with the help of fine capillary tube and transferred to glass tank filled with aquaculture wastewater which was collected from the aquaculture farm near Mimisal, Pudukottai District (South East coast of India). |
| B. Analysis of Water Quality Parameters – Initial Level (Before Copepod Inclusion) |
| The water quality parameters such as salinity, temperature, pH, nutrients, heavy metals and microbial population such as THB (Total Heterotrophic Bacteria), TVC (Total Vibrio Count) and TAC (Total Actinomycetes Count) were monitored before the copepod inclusion into the aquaculture wastewater. |
| C. Maintenance |
| The pH was measured using a pH meter. Water temperature was measured using a Celsius Thermometer. The salinity was recorded using a hand portable Refractometer (HR -150, SN 263716, Itally). Ammonia, nitrite, nitrate, phosphate, and silicate were estimated according to the method of Strickland and Parsons (1972). Heavy metals were analyzed by using atomic absorption spectrophotometer (CECRI, Karaikudi). |
| D. Microbial Population |
| The microbial population in the aquaculture wastewater was estimated according to the standard protocols. Water samples were serially diluted and spread on Zobell’s marine agar medium (Hi-Media, Mumbai, India) for total heterotrophic bacteria count, TCBS ( Thiosulfate Citrate Bile Salts Sucrose) medium for total Vibrio count and AIA (Actinomycetes Isolation Agar) for total actinomycetes count. |
| E. Inclusion of Copepods in Aquaculture Wastewater |
| After evaluating the water quality parameters and microbial population level, the 20 adult gravid female copepods were inoculated into aquaculture wastewater. Mild aeration was given during the culture. The experimental duration for the culture of Nitokra affinis was up to 21 days. During the period, the population growth of copepod Nitokra affinis was monitored. |
| F. Analysis of Copepod Population and Water Quality Parameters- Final Level (21 Days after Copepod Inclusion) |
| Copepod population was counted with the Sedgewick - Rafter counting cell using a binocular microscope (Olympus CH20i) after 21days. The nauplii, copepodites and adults were calculated as org/lit. The water quality parameters such as salinity, temperature, pH, nutrient, heavy metal analysis and microbial population such as THB (Total Heterotrophic Bacteria), TVC (Total Vibrio Count) and TAC (Total Actinomycetes Count) were once again monitored after 21 days of copepod cultured in wastewater. |
III. RESULTS AND DISCUSSION |
| Culturing of copepods with detritus organic matter has not given much importance because using phytoplankton (algae) as a feed for copepods has been used as the standard techniques for the culture of copepods [12], [22]. Although harpacticoid copepods are detritivorous, studies on copepod culture using inert diets are still been inadequate. Harpacticoid copepod can be reared on a variety of inert diets [18]. Copepod adults and nauplii consumed waste organic matter because waste organic matter (detritus) accumulates 5 times more than the phytoplankton biomass in the marine environment [13], [14]. For the successful copepod culture, attempts were made to develop intensive culture techniques. In our study, we made an attempt to culture Nitokra affinis, in the aquaculture wastewater. |
| A. Water Quality Parameters |
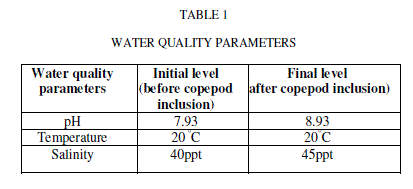
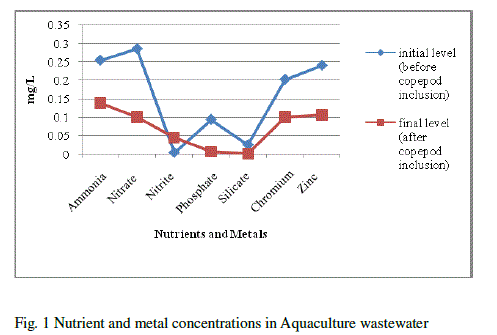
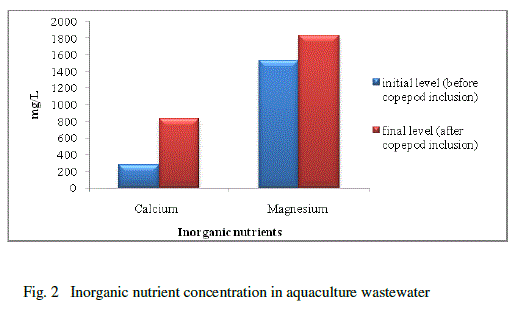
| Water quality parameters were shown in Table-1. There was no significant difference found in temperature. The pH showed variation during the cultured period. Its initial level ranged from 7.93 to final level of 8.93.There was a significant difference found in the salinity varied from 40 to 45ppt. The nutrients and heavy metals showed gradual decrease from initial value to final value especially ammonia declined from 0.255 to 0.138mg/L, nitrate 0.286 to 0.100 mg/L phosphate 0.094 to 0.006 mg/L, silicate 0.025 to 0.001mg/L, Zinc 0.241to 0.106mg/L and Chromium 0.203 to 0.100 mg/L but nitrite, calcium and magnesium showed a gradual increase from initial to final level (nitrite 0.004 to 0.045 mg/L, Calcium 276.05 to 833.5 mg/L and magnesium 1533.6 to 1831.7 mg/L) respectively. Surajit Das et al., [21] reported that declined in the ammonium level in the experimental tanks might be due to the increase in probiotic cell concentration which converts ammonia into nitrate by nitrification through the intermediary product nitrite. Our present findings showed that reduced ammonium level might be due to the presence of probiotic microbes which enhanced copepods for their growth and population in aquaculture waste water. The build-up of calcium and magnesium might be due to the addition of lime and pesticides in aquaculture wastewater for better production which was reported earlier by Mishra et al. [9]. This was coinciding in our result for the increased concentration of calcium and magnesium. |
 |
 |
| B. Microbial Population |
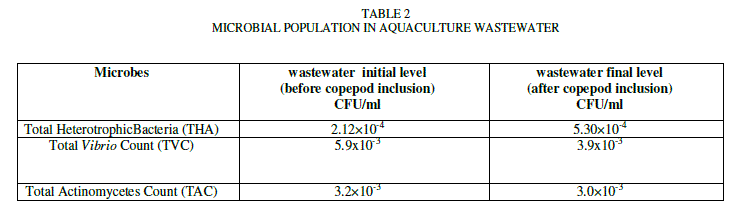
| The results of microbial population analysis were shown in Table-2. Microbial population in wastewater for total heterotrophic bacteria, pathogenic bacteria (Vibrio) and actinomycetes were reported. The result showed that there was a gradual decrease in population of actinomycetes (3.2×10-3 to 3.0×10-3 CFU/ml) and pathogenic bacteria (Vibrio) (5.9x10-3 to 3.9x10-3) CFU/ml from the initial level (before copepod inclusion) to final level (after copepod inclusion). Total heterotrophic bacteria showed a gradual increase (2.12×10-4 to 5.30×10-4 CFU/ml) during the cultured period. |
| Decline in the population of gram positive bacteria (actinomycetes) might be due to its utilization by copepod as a feed. Our findings agrees with Rieper [17]who reported that bacterial biomass and detritus were the superior source of food for the egg hatching rate and population in the growth of harpacticoid copepods. The heterotrophic bacteria population showed significant increase in our study. It played a major role through mineralization, decomposition of wastes and also provides feed for copepods. The similar study was reported by Sunilkumar [20] in which heterotrophic bacteria provide supplementary feed for shrimp larvae. Surajit das et al. [21] reported that beneficial bacteria (probiotics) enhanced in sustaining water quality, thereby improving survival and growth of shrimp. In our study, the probiotic microbes decreased the concentration of nutrients and metals. The pathogenic bacteria (Vibrio) the natural microflora of shrimp [8] can cause disease and mass mortality in the shrimp showed a significant decrease in our study. The cause of the decline might be due to the presence of beneficial microbes limiting the establishment of pathogenic bacteria [15], [16]. Probiotics inhibit pathogens by supplying vitamins (vitamin C, vitamin B12, retinol and others), proteins, amino acids and highly unsaturated fatty acids [5],[6],[23][3] for the host. Probiotic administration in the host stimulates immune response against pathogenic micro organisms [1], [7]. Beneficial bacteria present in wastewater are responsible for decomposition of unutilized feed and decay of biotic materials which are responsible for improvement in the growth of shrimp [21]. Obviously, in our findings these pathogenic bacteria did not seem to affect copepod population because probiotic microbes may be enhanced in stimulating immunity to the copepods which might be responsible for the improvement in the survival and population. The same trend reported by [1] and [7] in shrimp (Penaeus monodon and Peneaus vannamei) showed that good improvement in survival and growth could be due to the immunity stimulated by probiotic microbes. |
 |
| C. Copepod Population |
| In the first day, 20 healthy gravid females of adult Nitokra affinis were inoculated in shrimp wastewater. Mild aeration was given. Over 21 days cultured period, the cultured system produced an average of 650ind/lit (nauplii- 350 ind/l, copepodite- 200 ind/l and adults- 100 ind/lit) were shown in the Fig. 3. |
 |
IV.CONCLUSION |
| Survival and increased growth of copepod (Nitokra affinis) in aquaculture wastewater might be due to the presence of probiotic microbes. Sun et al. [19] recently reported in calanoid copepod Pseudodiaptomus annandalei can performed as a suitable probiotic vector for the larvae Epinepheluscoioides. They also found that fish larvae fed with probiotic induced copepod, resulted in good improvement in growth. Therefore, in future several copepod vectors (Probiotic) are warranted in aquaculture to maximize the production and to improve the growth in shrimp larvae. Hence, our present finding could support additional evidence for applying probiotic vector using harpacticoid copepod (Nitokra affinis) as a livefeed for aquaculture in future.The development of mass culture of Nitokra affinis in aquaculture waste water revealed that this species grows well using relatively simple culturing techniques. With the improvement of these techniques and studies on the probiotics administration for the fish larvae via copepods even more satisfactory results could be achieved, thus increasing the highly nutritional live feed for aquaculture. The present investigation revealed that Nitokra affinis could be culture in aquaculture wastewater and probiotic microbes influenced the survival and population growth of Nitokra affinis. |
References |
|