ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| P. Naveen Kumar*, U. Sasikala, and A.K. Sharma *Department of Physics, S.V. University, Tirupati-517502, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Sodium ion conducting polymer electrolyte films based on Poly (ethylene oxide) PEO/Poly (ethyl methacrylate) PEMA have been prepared by solution-cast technique in various concentrations. Miscibility studies of the electrolytes were investigated by X-ray diffraction (XRD) and the results show that the highest conducting film is the most amorphous. Frequency dependent conductivity (σac) values were obtained from complex impedance (colecole) plots. It was observed that the magnitude of conductivity increased both with the increase in the salt concentration and the temperature. The charge transport of these electrolytes is mainly due to ions which were confirmed by the transference number experiment. Using this electrolyte, cells were fabricated and their discharge profiles were studied under constant load. Several cell parameters associated with the cells were evaluated and compared with earlier reports.
Keywords |
| Polymer blend electrolyte, XRD analysis, AC conductivity studies, Transference numbers, Discharge profiles. |
INTRODUCTION |
| In the recent years, polymer electrolytes have been widely studied due to their applicability for a variety of solid state and electrochemical device applications including batteries, fuel cells, supercapacitors, electrochromic devices and chemical sensors [1]. Polymer electrolytes have many advantages, such as flexibility, ease of processing into thin films of large surface area, electrochemical stability and leak-proof nature [2]. Various approaches such as blending [3], [4], co-polymerization [5], plasticization [6], addition of ceramic fillers [7] etc. have been made to enhance the ionic conductivity of polymer electrolytes [8]. For the present study, we have adopted one of the above techniques, namely, blending because of the ease of preparation and easy control of physical properties within the compositional regime, which thus reduces development costs. The inherent merits of using blend-based polymer electrolytes have been exemplified by several research groups [3], [4] & [9]. In the present study, the effect of doping with sodium perchlorate (NaClO4) on electrical conduction in (PEO+PEMA) polymer blend electrolyte films has been undertaken. Several experimental techniques such as XRD, electrical, transference numbers and discharge characteristics were performed to characterize these polymer blend electrolytes. |
II. EXPERIMENTAL |
| Films (thickness~150 μm) of (PEO+PEMA) blend and NaClO4 salt complexed (PEO+PEMA) blend were prepared in weight ratios (47.5:47.5:5), (45:45:10), (42.5:42.5:15) and (40:40:20) by solution cast technique using dimethylformamide (DMF) as the solvent. The X-ray diffraction studies of these films were performed by means of a SEIFERT X-ray diffractometer system with Ni-filtered CuKα radiation. The impedance measurements were performed using a complex controlled phase sensitive multimeter (PSM 1700) in the frequency range 1 Hz – 1 MHz and temperature range 303 – 373 K. The total ionic transport number (tion) was evaluated by means of Wagner’s polarization technique [10]. In this technique, freshly prepared polymer electrolyte films were polarized in the configuration Na/polymer electrolyte/C under a DC bias. The resulting current was monitored as a function of time. Electrochemical cells were fabricated with the configuration Na/(PEO+PEMA+NaClO4)/(I2+C+electrolyte). The discharge characteristics were monitored under a constant load of 100 kΩ. |
III. RESULTS AND DISCUSSION |
| A. X-ray diffraction studies XRD studies were made on pure (PEO+PEMA), NaClO4 and their complexes (Fig. 1) in order to investigate the complexation and influence of the concentration of NaClO4 salt. The diffraction peaks of pure PEO was observed at 2θ = 19.1°, 23.3° and that of PEMA at 2θ=16.5°. The intensity of these plots decreased in pure (PEO+PEMA) blend. Also while increasing the salt concentration in the polymer blend the intensity of the peaks significantly decreases. The disappearance of NaClO4 peaks in polymer complexes indicates the complete dissolution of the salt in the polymer matrix. The decrease in the intensities of peaks showed that the addition of salt causes a decrease in degree of crystallinity and simultaneous increase in the amorphosity of the complexed films. |
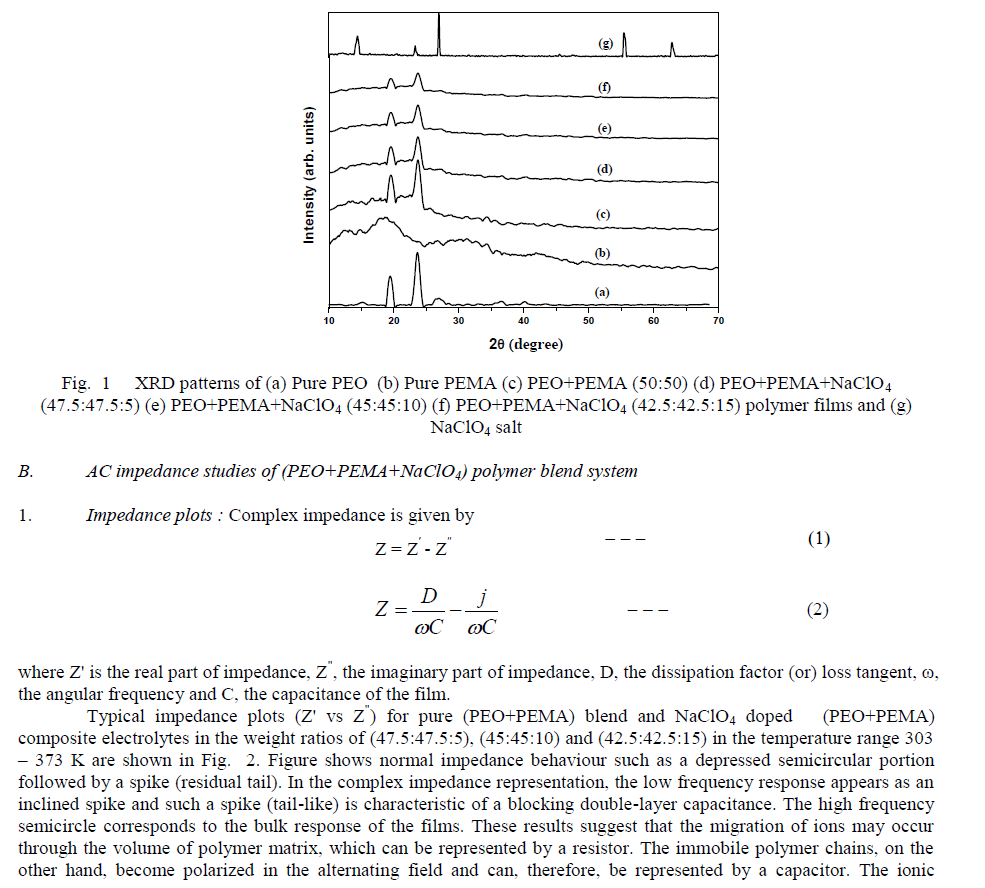 |
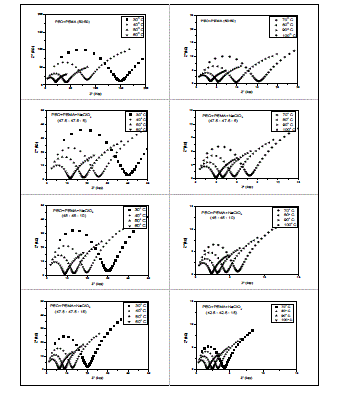
| migration and bulk polarization are physically in parallel and, therefore, the portion of the semicircle can be observed at high frequencies [11]. The ionic conductivity of (PEO+PEMA) and (PEO+PEMA+NaClO4) polymer electrolytes is calculated from the relation where I, is the thickness of the film, A, the area of the film and Rb, the bulk resistance of the film material which is obtained from the intercept on the real axis at the high frequency end of the Nyquist plot of complex impedance [12]. 2. Composition dependence of conductivity : The variation of conductivity (σ), as a function of NaClO4 salt concentration in (PEO+PEMA) at different temperatures is shown in Fig. 3. From the figure, it is seen that the conductivity of pure (PEO+PEMA) is about 8.30 X 10-8 Scm-1 at room temperature and increases to 3.22 X 10-7 Scm-1 for 5 wt% NaClO4. The increase in conductivity becomes flatter on further addition of NaClO4 to the polymer upto 15 wt%. The increase in conductivity at lower dopant concentrations of NaClO4 is attributed to the formation of charge transfer complexes or decrease in the crystallanity [13], while the slow increase at higher dopant concentrations is due to the formation of ionic aggregates. For further increase of the salt concentration (20 wt% of NaF to the polymer) the conductivity decreases. This may be due to the increasing influence of the ion pairs, ion triplets and higher ion aggregations, which reduce the overall mobility and degree of freedom [14]. The conductivity data of pure and complexed (PEO+PEMA) at room temperature are presented in Table 1. |
 |
| Fig. 2 Impedance (Cole-Cole) plots of (PEO+PEMA) blend and (PEO+PEMA+NaClO4) polymer blend films at different temperatures |
 |
| Fig. 3 Composition dependence of conductivity of (PEO+PEMA+NaClO4) polymer electrolyte system at room temperature |
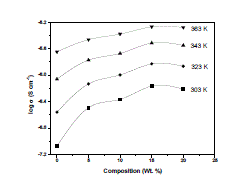
| 3. Temperature dependence of conductivity : The bulk conductivity has been calculated from the impedance polt, and logarithms of conductivity were plotted against the inverse of temperature to observe the temperature dependence of conductivity. Fig. 4 shows the linear dependence of logσ on inverse temperature (1000/T) for pure and complexed films. As per Arrhenius relation the dependence of conductivity has the form exp( ) 0 E kT a -------- (4) where σ0 is the pre-exponential factor, Ea, the activation energy and, k, the Boltzmann constant. The magnitude of ionic conductivity was found to increase with increase in temperature in all compositions of the polymer blend electrolyte system including pure film. This may be explained on the basis of an increase in either ionic mobility or the concentration of carrier ions [15]. |
 |
| Fig. 4 Temperature – dependent conductivity of (a) Pure PEO+PEMA (b) PEO+PEMA+NaClO4 (47.5:47.5:5) (c) PEO+PEMA+NaClO4 (45:45:10) (d) PEO+PEMA+NaClO4 (42.5:42.5:15) (e) PEO+PEMA+NaClO4 (40:40:20) polymer films |
| In polymer electrolytes, change of conductivity with temperature is due to segmental motion, which results in an increase in the free volume of the system [16]. When temperature is increased, the vibrational energy of a segment is sufficient to push against the hydrostatic pressure imposed by its neighbouring atoms and create a small amount of space surrounding its own volume in which vibrational motion can occur [15]. Therefore, the free volume around the polymer chain causes the mobility of ions and polymer segments and, hence, the conductivity increases. The increase of temperature causes the increase in conductivity due to the increased free volume and their respective ionic and segmental mobilities. This increase in free volume would facilitate the motion of ionic charges [17]. 4. Activation energies : The activation energies were calculated from the slope of the Arrhenius plots and the values are shown in Table 1. The activation energy is a combination of energy of charge carrier creation (defect formation) and the energy of ion migration that can be evaluated by linear fitting of the log σ vs 1000/T plots [18], [19]. Therefore, it can be suggested that the activation energy is due to the energy that is required to provide a conductive condition for the migration of ions. From the table it was found that the activation energy values decreased with increasing concentration of NaClO4. This may be due to the fact that the addition of small amounts of dopant forms charge transfer complexes in the host lattice [20]. These charge transfer complexes increase the electrical conductivity by providing additional charges in the lattice, resulting in a decrease of activation energy. |
 |
| C. Transference number |
| In Wagner’s polarization technique dc current was monitored as a function of time on application of fixed dc voltage across the Na/polymer electrolyte/C. After polarization of the cell with 1.5 V dc, the current versus time plots were obtained and shown in Fig. 5. The transference numbers (tion and tele) were calculated from the polarization current versus time plots using the equations |
 |
| where Ii is the initial current and If, the final residual current. |
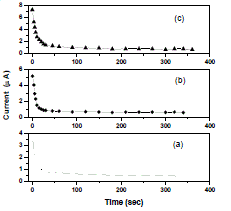 |
| Fig. 5 Current vs time plots of (a) PEO+PEMA+NaClO4 (47.5:47.5:5) (b) PEO+PEMA+NaClO4 (45:45:10) (c) PEO+PEMA+NaClO4 (42.5:42.5:15) polymer blend electrolytes |
| The resulting data is given in Table 2. For all the compositions of (PEO+PEMA+NaClO4) electrolyte system, the values of ionic transference numbers are in the range 0.89 - 0.91. This suggests that the charge transport in these polymer electrolyte films is predominantly due to ions; only a small fraction of contribution (0.11 – 0.09) comes from the electrons [21]. Table II Transference numbers data of (PEO+PEMA+NaClO4) polymer blend electrolyte system |
 |
| Furthermore from the table it is clear that the increase of salt concentration has significant effect on the transport properties of the electrolytes. The ion transport number increases with the increase of salt concentration. This is due to the enhancement of ionic concentration (both cationic and anionic) which results in high initial current. The ion transport number reached a high value for 15 wt% of NaClO4 complexed PEO+PEMA+NaClO4 polymer electrolyte system. D. Discharge profiles In order to get better performance and stability of cell parameters (PEO+PEMA+NaClO4) polymer blend electrolyte film cells were fabricated in the configuration Na (anode)/ (PEO+PEMA+NaClO4)/(I2+C+electrolyte)(cathode) for various compositions of (PEO+PEMA+NaClO4) with ratios (47.5:47.5:5), (45:45:10) and (42.5:42.5:15). Their discharge profiles were studied for a constant load of 100 kïÃÂÃâ at room temperature. Fig. 6 shows the discharge characteristics of these polymer batteries. From the figure it was found that during discharge, the cell voltage decreases initially, and then remains constant for a particular duration (time of stable performance of the cell) after which voltage declines. The initial sharp decrease in voltage may be due to the activation polarization and/or the formation of thin layer of sodium at electrodeelectrolyte interface [22]. The evaluated cell parameters from the discharge characteristic such as OCV, SCC, current density, plateau region time, power density, energy density etc are given in Table 3. |
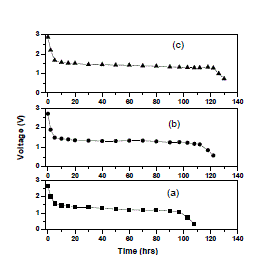 |
| Fig. 6 Discharge characteristics of (a) PEO+PEMA+NaClO4 (47.5:47.5:5) (b) PEO+PEMA+NaClO4 (45:45:10) (c) PEO+PEMA+NaClO4 (42.5:42.5:15) polymer blend electrolytes |
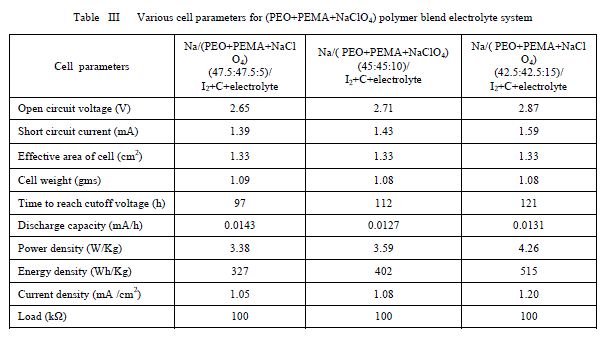 |
| It was observed that all the parameters showed an increasing trend with increasing percentage of NaClO4 in the (PEO+PEMA) polymer blend. From all the compositions (PEO+PEMA+NaClO4) (42.5:42.5:15) cell showed better performance and stability. This may be due to the higher ionic conductivity and greater amorphosity of this polymer electrolyte system. |
IV. CONCLUSIONS |
| A new Na+ ion conducting (PEO+PEMA) solid polymer blend electrolyte membrane: (PEO+PEMA+NaClO4) (42.5:42.5:15) has been synthesized. The impedance study showed that the addition of salt to the polymer electrolyte enhanced the ionic conductivity. The transference number measurements indicated that the newly synthesized polymer electrolyte is an ionic conductor and the charge transport is due to ions. These polymer blend electrolytes exhibit better performance, which indicates that such electrolytes are more suitable for fabricating solid-state batteries. |
References |
|