ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
G. Krithika1, P. Manasa Satheesh2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Developments in the Biofuel production has created more awareness in Research due to the rapid increase in oil price and growing environmental issues. Microalgae have gathered attention towards them in the Biofuel production. Transesterification process converts algal oil or lipids to fatty acids. Because of their high Biomass and Lipid accumulation, the microalgae suited for the production of bio oil in industrial level. The lipid content was 28.22±1.38%, carbohydrate 16.8% and protein 58.2% for Chlorella pyrenoidosa. The amount of oil extracted was found to be 25.8 ml for (Alternate Media) AM1 and 20.6 ml for AM2. The pH was found to be 7.8 for AM1 and 7.6 for AM2. In this study, scaling up of Chlorella pyrenoidosa in poultry waste water was done with fertilizers and analysingparameters for its growth and production of Bio oil from Chlorella pyrenoidosa has been examined from the scaling up in poultry waste water
Keywords |
| Bio oil, Lipids, Carbohydrates, Chlorella pyrenoidosa, Proteins, Transesterification, Wastewater. |
INTRODUCTION |
| Microalgae are sunlight-driven cells that convert carbon dioxide to potential biofuels, foods, feeds and high-value bioactives. Microalgae could provide alternatives to petroleum based fuels without competing with crops. Microalga is an economical and potential raw material of biomass energy [12]. Chlorella pyrenoidosa is a unicellular green alga that grows in fresh water. C. pyrenoidosa contains much more protein and chlorophyll than other plants. Spirulina, like any blue-green algae, can be contaminated with toxic substances called microcystins. It can also absorb heavy metals from the water where it is grown [7]. Chlamydomonas is the algae from genus of microscopic, unicellular green plants (algae) which live in fresh water. It has been found that they can be used to generate hydrogen from light, water, and basic nutrients. The possibility of generating large quantities of hydrogen, which is a renewable fuel, from cheap and abundant sources is produced by Chlamydomonas [11].A critical growthâÃâ¬ÃÂlimiting factor is the carbon, usually supplied as CO2 that occurs in enriched environments. Supply of pure CO2 into algae cultivations is expensive and not an encouraged option for large scale algae production. However, through a lower cost option, algae have the ability to capture CO2 from combustion gas and from natural gas operations and hold the opportunities to recycle CO2 [15]. The transesterification process is the reaction of a triglyceride (fat/oil) with an alcohol to form esters and glycerol. A triglyceride has a glycerine molecule as its base with three long chain fatty acids attached [4].Almost all biodiesel is produced in a similar chemical process using base catalysed transesterification as it is the most economical process, requiring only low temperatures and pressures while producing a 98% conversion yield. Biodiesel is, at present, the most attractive market alternative among the non-food applications of vegetable oils for transportation fuels. Bio oil can be used in pure form. Bio oil higher lubricity index compared to petroleum diesel and can contribute to longer fuel injector life. Bio oil is pollution free solvent than petroleum diesel and has been known to break down deposits of residue in the fuel lines of vehicles that have previously been run on petroleum diesel. Chemical solvents are often used in the extraction of the oils [10]. In this study the growth of Chlorella pyrenoidosa is scaled up from the results obtained by various trials and the extraction of bio oil is done successfully by transesterification process. |
II. MATERIALS AND METHODS |
| Collection of algal strains and Waste water: The algal strains such as Chlorella pyrenoidosa, Spirulina and Chlamydomonaswere collected from NCIM, Pune and maintained properly in its selective medium at Genewin Biotech, Hosur. Poultry waste water was obtained from Maruthi Hatcheries, Hosur and Tannery waste water obtained from a leather industry Vanitech, Vaniyambadi. Determination of Lipid Content in the Three Algae Species: Extraction and determination of intracellular lipids were conducted according to the standard protocols in the previous literatures [1]and[3]by Gravimetry.Freeze-dried algal biomass (50−100 mg) were extracted three times with methanol containing 10% DMSO (volume fraction) for 50 min under stirring. Then the mixture was centrifuged (2000 g, 10 min), and the supernatant was removed. The residua were re-extracted twice with a mixture of hexane and diethyl ether (1:1, volume ratio) for 30 min. After extractions, hexane and water were added to the combined supernatants, so as to obtain a ratio of 1:1:1:1 (volume ratio) for the above four solvents. The mixture was shaken and then centrifuged for 10 min and the upper organic layer was collected. After the water phase was re-extracted twice with a mixture of hexane and diethyl ether (1:1, volume ratio), the organic phases were combined and evaporated to dryness. Then it was redissolved with a small amount of hexane. The lipid solution was transferred into a pre-weighed vial, initially evaporated in a water bath (30 °C) using a rotatory evaporator and then dried under high vacuum. The dried residua were weighed on an electronic microbalance. Determination of Protein Content in the three Algae Species The Lowry method was used to measure the protein content of the pre-treated biomass [2]and[16].The absorbance of the sample was measured at a wavelength of 750 nm in a spectrophotometer [5]. Reagents Alkaline copper sulphate solution was prepared freshly by mixing 1.0 mL of 0.5% CuSO4.5H2O in 1% Potassium sodium tartarate with 50.0 mL of 2% sodium carbonate in 0.1 N NaOH. Diluted Folin-phenol reagent was prepared by diluting Folin-ciocalteu reagent obtained with an equal volume of glass distilled water. Procedure To one millilitre of protein solution, 5.0 ml of alkaline copper sulphate solution was added and allowed to stand at room temperature for 10 minutes. Then 0.5 ml of diluted Folin-phenol reagent was added and mixed well[8]. The solution was allowed to stand for 30 minutes and absorbance was read at 500 nm using a Hitachi UV 2001 spectrophotometer. BSA (Sigma fraction V) was used as standard. The protein content of the biomass was calculated using the following equation: Protein (%, w/w) = CVD/m x 100 where C is the protein concentration (mg L-1) obtained from the calibration curve, V is the volume (L) of the lysis buffer used to resuspend the biomass, D is the dilution factor and m is the amount of biomass (mg). Determination of Carbohydrate Content in the Three Algae Species Carbohydrates were extracted and estimated as the procedure given by Roe and Indian Pharmacopeia. Freshly harvested, frozen thalli were used within two days of collection. Known quantity of the alga was ground in a glass mortar and pestle with 80% ethanol and filtered through Whatman’s No. 1 filter paper. The filtrate was centrifuged at 8000 X g for 20 minutes and the supernatant was made to 5.0 ml with 80% ethanol. The final volume of the extract was then made up to 10.0 ml by adding distilled water. To 1.0 ml of the above sample, 4.0 ml of anthrone reagent was added by the sides of the test tube and heated in a boiling water bath for 10 minutes. The tubes were cooled to room temperature and absorbance was read at 620 nm using UV spectrophotometer. A reagent blank was run simultaneously. Glucose was used as the standard [9]. |
| Anthrone reagent Anthrone (50.0mg) and thiourea (1.0 g) were added to 100.0 ml of 72% cold sulphuric acid and stored in a dark bottle. Freshly prepared reagent was used for every estimation. Trials done with Waste water and Fertilizers: Two types of waste water were selected and trials were put with the Alternate media (AM1 and AM2) which are fertilizers available in the following ratios of N, P and K: ïÃâ÷ AM1: 0:52:34 (supplementing nitrogen with Urea) ïÃâ÷ AM2: 13:0:45 (supplementing P with super phosphate) Various parameters necessary are measured in TWW and PWW for the growth of algae. Chlorella pyrenoidosawas trialled with various ratios with AM1 and AM2 fertilizers at pH 6, 7, 8. It was clearly seen that the TWW did not promote growth of Chlorella as the ratio of TWW was higher than the fertilizers. So the experiment was further continued with PWW. Altering the biological parameters of TWW, the Chlorella can be grown in the TWW with the various ratios along with the fertilizers supplementation.PWW was used for the growth of Chlorella once the biological parameters are analysed. Various trials such a PWW: AM1 and PWW: AM2 with the ratios 0:50, 50:0, 30:20, 20:30, 40:10, 10:40 were put using two different fertilizers for checking the suitable combination of ratio for the growth of Chlorella pyrenoidosa. |
Estimation of Growth of Chlorella with CO2: |
| As with all photosynthetic organisms, algae use CO2 as a carbon source. No growth can occur in the absence of CO2. Based on the average chemical composition of algal biomass, approximately 1.8 tonnes of CO2 are needed to grow 1 tonne of biomass. Method: The CO2 coming out from a tall chimney was collected and sent in through a motor which directly sent the CO2to the algae bottle. Side by side control with the aerators where kept and corresponding OD values recorded at intervals of 1 hrs. each [6]. |
Bio oil Production: |
| The collected Chlorella was expanded in the Fog’s medium. The factors necessary for the cultivation of algae are Light, Temperature, and Nutrients. The algae which was cultivated was grown and then expanded in the 25 liters Bubble tops inorder to achieve the mass production of algae. The medium used for the growth of Chlorella was Fog’s medium. The algae was grown in the Bubble tops and the algal biomass was collected for the oil extraction. Flocculation is a reliable method for algae separation. The effect of culture pH on the flocculation efficiency was carried out by adjusting the culture pH ranging from pH 10 to pH 10.6 using either 5 M sodium hydroxide (NaOH) or 5 M potassium hydroxide (KOH). The bases were added to the culture by agitation using magnetic bar stirrer (38 mm), agitated at 200 rpm to allow for steady increase in pH [14].Biomass biologically is the material of plants which are specifically called lignocellulosic biomass. The biomass was collected after filtration and weighed.Algae were ground with motor and pestle and the ground algae were dried for 20 min at 80°C in a incubator for releasing water. Hexane and ether solution (20 and 20 mL) were mixed with the dried ground algae to extract oil. Then the mixture was kept for 24 h for settling. |
Transesterification Process: |
| The transesterification reaction is base catalysed. The presence of water causes undesirable base hydrolysis, so the reaction must be kept dry bytransesterification process. The conical flask containing solution was shaken for 3 h by electric shaker at 300 rpm.After shaking the solution was kept for 16 h to settle the biodiesel and sediment layers clearly.The bio oil was separated from sedimentation by flask separator carefully. Biodiesel was washed by 5% water until it was became clean since the washing can eliminate the unwanted impurities and removes all sorts of debris from the mixture. |
| 0.25 g NaOH was mixed with 24 ml methanol and stirred continuously for 20 minutes. The mixture of catalyst and methanol was poured to the algal oil in a conical flask and tranesterification process allowed to take place.The bio-oil is separated from the sedimentation by a flask separator. Bio-oil was washed with 5 % sterile water until it becomes clean. Bio-oil dried by using a dryer and kept under air for 12 hrs. Harvesting has been claimed to contribute 20–30 percent to the total cost of producing the biomass [6].Bio oil production was measured by using measuring cylinder; pH was measured and stored for analysis. |
III.RESULTS AND DISCUSSION |
Determination of Lipids,Proteins and Carbohydrates |
| Lipids are produced during physiological experiments and biomass production processes. Here, the lipid content is estimated for three species.The lipid content was found to be high in Chlorella pyrenoidosa of 28.22±1.38 %followed by Spirulina of 24.00±4.49 %and Chlamydomonas15.61±0.63%.Proteins make up a large fraction of the biomass. The Lowry method is one of the most accurate methods for quantifying proteins [5]. Bovine serum albumin (BSA) is the most commonly used standard. The protein content was found to be highest in Chlorella pyrenoidosa of 58.2% followed by Spirulina of 52.6% and Chlamydmonas of 48%. Determination of carbohydrate concentrations is the colorimetricmethod based on reaction between hydrolysed carbohydrate solution and a colouring reagent that develops colour that is detectible in the visible range of the electromagnetic spectrum. Carbohydrate content was highest in Chlorella pyrenoidosa of 16.8% and lowest in Spirulina of 12.4%. |
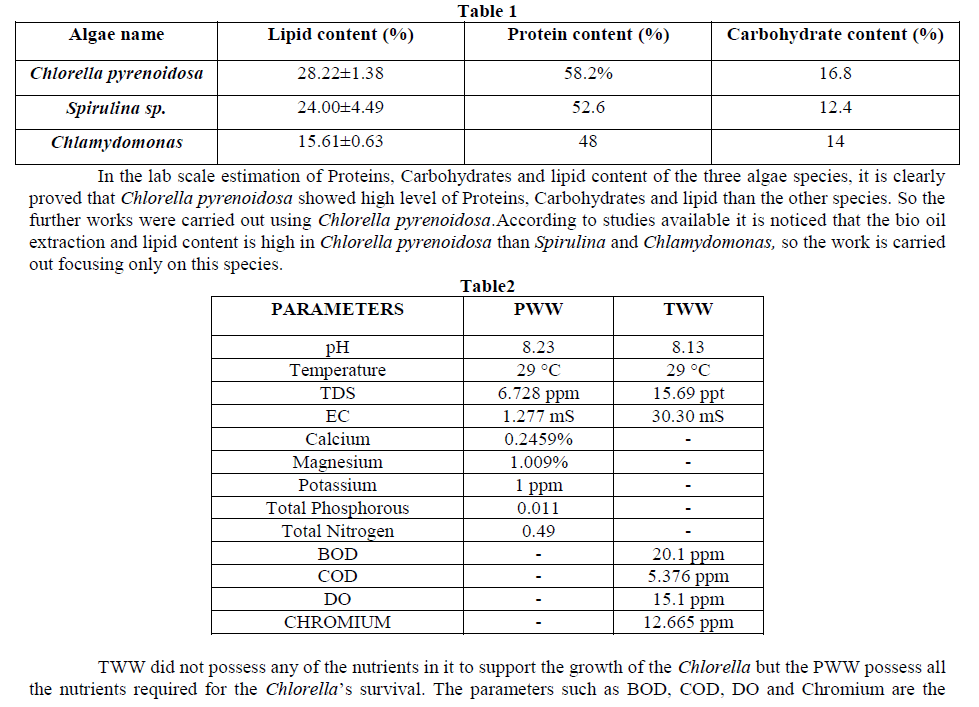 |
| parameters specific for the water analysis to be used and it is not related with the growth of Chlorella. SO, PWW was used for the further trials and Bio oil production. |
CARBON DIOXIDE EXPERIMENT FOR THE ALGAL GROWTH |
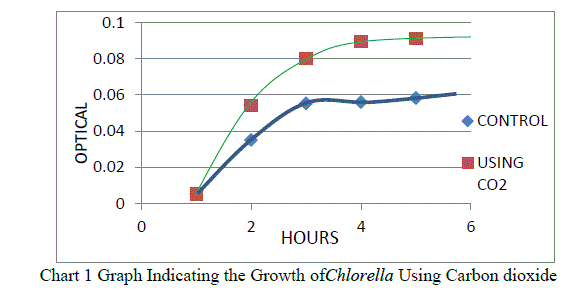
| Carbon dioxide experiment was conducted in order to check the growth of Chlorella using carbon dioxide. A control was also checked without using the carbon dioxide. The absorbance was taken for a period of 5 hours to check whether Carbon dioxide is helping the Chlorella growth[13]. |
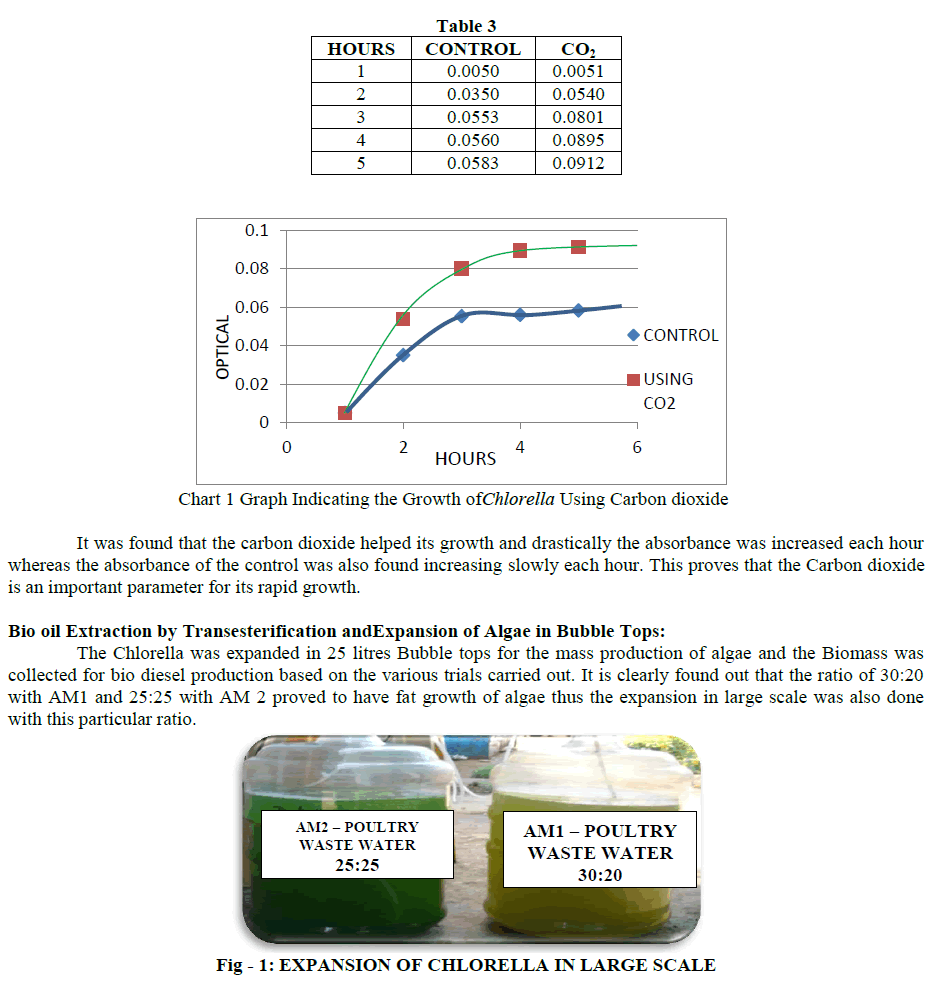 |
| The expansion was done in the above ratio in the bubble tops and the growth of Chlorella was seen in both the ratios. It took about 1 week for the Chlorella to reach its maximum growth rate. Scaling Up in Bubble Tops: The scaling up of the PWW was done with AM1 fertilizer in the ratio 30:20 and the Chlorella was allowed for expansion. It took 10 days for the Chlorella to reach its maximum growth. The scaling up of the PWW was done with AM2 fertilizer in the ratio 25:25 and the Chlorella was allowed for expansion. It took 10 days for the Chlorella to reach its maximum growth. |
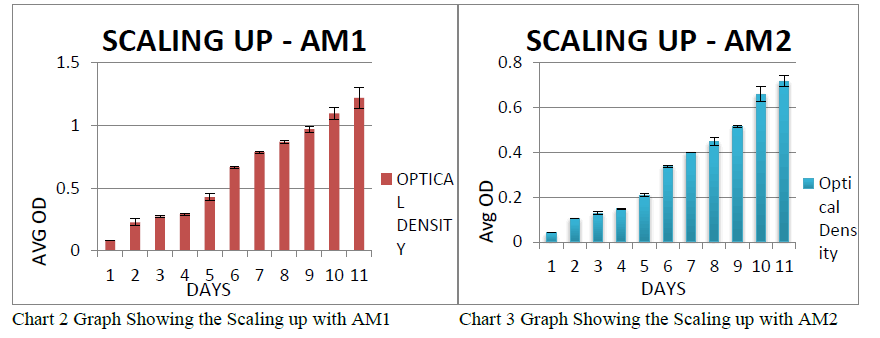 |
| It was observed that the mean absorbance was 1.463±0.0039 on the 10th day which showed the efficiency of AM1 in PWW.It was observed that the mean absorbance was 0.71815±0.0239 on the 10th day which showed that AM2 was not much efficient in PWW when compared to AM1. Flocculation: The Flocculating agent used was Aluminium Chloride for the separation of algae and the water in the bubble top for the collection of biomass. The Flocculation involved the separation of species by gravity. The various techniques used for flocculating were decreasing the pH to 4 using acid, increasing the pH to 11 using alkali, Addition of ferrous sulphate, Addition of aluminiumsulphate, Addition of ferric chloride. It is found that decreasing the pH to 4 using acid seemed to help in flocculation faster followed by aluminium chloride. Trials were put and then concluded as to which method to flocculate algae in large scale production. Algae were collected and dried for 20 minutes at 80 °C in an indicator for releasing water. Hexane and ether solution (20 ml each) were mixed with the dried ground algae to extract oil. The mixture was kept for 24 hrs. for settling. The Biomass was collected after the Flocculation process and the collected biomass was weighed. |
 |
| The collected Biomass cake obtained from AM1 after drying was observed as 21 g which was higher when compared to AM2 and this was further used for carrying out Bio oil production. |
 |
IV. CONCLUSION |
| It was concluded that the Bio oil can be produced from the algal species efficiently which was environmental friendly and Pollution free. Algae species which is known for its high availability in nature, renewable energy source and low cost, it could be used to produce Bio oil in an inexpensive manner. In this study, the oil was produced from the algae efficiently by Transesterification. The amount of oil extracted was found to be 25.8 ml for AM1 and 20.6 ml for AM2. The pH was found to be 7.8 for AM1 and 7.6 for AM2. By this way, Chlorella pyrenoidosa can be used as renewable energy source. |
References |
|