ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sunaina Zutshi1, Fareha Bano2, Manikar Ningthoujam2, Khalid Habib2 & Tasneem Fatma3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Arsenic is the most common nonessential widely occurring metal with serious hazardous impacts on living organism. Arsenic enriched soils are considered major sources of contamination in the food chains and water supplies. Presence of arsenic is of great environmental concern due to its carcinogenic and mutagenic property in addition to high toxicity. Very little work is done on arsenic induced toxicity and resistance in cyanobacteria who are main producer of aquatic system. Thus during present investigation, in vitro study was done to determine the toxic effect of Sodium Arsenate (0- 100mM) on an aquatic cyanobacteria Hapalosiphon fontinalis-339 with respect to lipid peroxidation, ion leakage, photosynthetic pigments, antioxidant enzymes and non enzymic oxidative substances. 50% decrease in growth (LC50) was observed at 70 mM sodium arsenate. A marked reduction in photosynthetic pigments was observed in test organism at sublethal concentration (100mM sodium arsenate). At this level MDA production was enhanced that probably resulted in decreased growth of the test organism. Accumulation of enzymic and non enzymic substance e.g. superoxide dismutase, catalase, and ascorbate peroxidase activiti
Keywords |
| Sodium Arsenate pollution, Oxidative stress, Hapalosiphon fontinalis-339, Electrolyte leakage, Antioxidant defence system. |
INTRODUCTION |
| Geochemical reactions and industrial waste discharges as well as agricultural use of pesticides are greatly responsible for arsenic contamination in the aquatic environment [1]. In India and Bangladesh, the natural concentration of arsenic in drinking water is >50 μg/L and may be as high as 400 μg/L in paddy fields. The United States Environmental Protection Agency (USEPA) has identified arsenic as a group A known “carcinogen.”To prevent adverse impacts of arsenic older limits >50μg/L, were lowered to 10 μg/L for both community and non-transient, non-community water systems [2]. Drinking of arsenic contaminated water is responsible for the development of hyper pigmentation, skin and liver cancer, circulatory disorders, and other ailments [3]. Arsenic (As) and its compounds are ubiquitous in nature and exhibit both metallic and non-metallic properties. The inorganic species [arsenite, As (III) and arsenate, As (V)] are of great concern in drinking water sources. The most common trivalent inorganic arsenic compounds are arsenic trioxide, sodium arsenite and arsenic trichloride. Pentavalent inorganic compounds include arsenic pentoxide, arsenic acid and arsenates (calcium arsenate, lead arsenate and sodium arsenate). Common organic arsenic compounds are arsanilic acid, methylarsenic acid, dimethylarsinic acid (cacodylic acid) and arsenobetaine. Sodium arsenates are highly soluble in water. Interchanges of valence state may occur in aqueous solutions, depending on the pH and on the presence of other substances which can be reduced or oxidized [4]. Marine algae such as Fucus, Ascophyllum concentrate arsenic and selenium to high levels relative to their environment, with concentration factors of 1,000 to 10,000 [5]. Several authors detected arsenic in the form of arsenosugars in different seaweed genera such as Sargassum, Undaria, Enteromorpha and Ulva [6-7]. Arsenic resistances have been reported in few cyanobacteria like Synechocystis sp. PCC 6803 [8] and Phormidium [9]. Effect of different factors on arsenic accumulation was studied in Dunaliella salina together with changes in cellular biochemistry [10]. Arsenic toxicity has been studied only in few cyanobacterium (Oscillatoria princepes, O. limosa, Anabaena sp., Phormidium laminosum) and Algae (Euglena & Navicula) [11]. The toxic effect of heavy metals and metalloids appears to be related to the production of reactive oxygen species. In Anabaena doliolum chlorophyll decreasesd due to toxicity of AsV [12]. To the best of our knowledge not much is known regarding the biochemical modulations of the antioxidant defense system of different cyanobacteria under arsenic stress. Therefore, during present investigation metabolic adaptation were studied in cyanobacterium Hapalosiphon fontinalis-339 with special references to arsenic induced oxidative stress and its defense. |
II. MATERIAL AND METHODS |
| A. Growth and Culturing Conditions H. fontinalis-339 was procured from the National Centre for Culture Collection and Utilization of Blue Green Algae, Indian Agriculture Research Institute, New Delhi. The cells were grown in BG-11 medium, with and without sodium arsenate [13] in 2000 ïÃâñ 200 lux light intensity, 12:8 h (light: dark) regime, 30 ïÃâñ 1ïÃâðC temperature. The cultures were shaken manually daily. The stock solution was prepared in DDW and sterilized through the Millipore membrane filter assembly (0.02μm). Cultures were exposed to Sodium arsenate (0, 25, 50, 75 and 100 mM) for different duration (24h, 32h and 72h) and the absorbance at (λ 650) were measured for growth. |
| B. Photosynthetic Pigments (Chlorophyll, Carotenoids, Phycobiliprotein) Chlorophyll a and carotenoid were extracted in 80% acetone from treated and untreated cyanobacterial cells and their amount was estimated according to the method of [14-15] respectively. The absorbance of the supernatant was observed at ïÃÂì650 and ïÃÂì665 against 95% methanol as blank and at ïÃÂì450 against 85% acetone as blank. Phycobiliprotein were extracted with 2 mL phosphate buffer (0.1 M, pH 7.0) after repeated freezing and thawing [16]. The absorbance of supernatant was observed at ïÃÂì562, ïÃÂì615 and ïÃÂì652. |
| C. Oxidative Damage (MDA and Electrolyte Leakage) Oxidative damage in cyanobacterial cells was measured by the method of [17] in terms of the total content of 2– thiobarbituric acid-reactive substances (TBARS) and expressed as equivalent of MDA (malondialdehyde). 50 mg culture was grounded in 3 ml of 0.1% (w/v) trichloroacetic acid (TCA) at 40 °C following centrifugation at 13,000 g for 2 min. An aliquot of 0.5 ml from the supernatant was added to 1.5 ml TBA (0.5% in 20% TCA). Samples were incubated at 95 ºC for 20 min and the reaction was stopped by cold treatment in ice bath. Centrifugation at 1000g for 5 min was performed and absorbance of the supernatant was measured at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. The concentration of MDA was calculated using its extinction coefficient (155 mM –1cm-1). |
| Electrolyte leakage was determined according to the method of [18]. Samples were rinsed with distilled water and immersed in 6 ml of distilled water for 12 h. The conductivity of the solution (R1) was measured with a conductivity meter (Model DDS-11A, Shanghai Leici Instrument Inc., Shanghai, China). Samples were then heated in boiling water for 20 min and then cooled to room temperature. The conductivity of killed cells (R2) was again measured. Electrolyte leakage was calculated as the ratio of R1- R2. |
| Relative Water Content Fresh biomass (0.5 g; Wf) were rinsed in water. The saturated biomass were weighed (WS) and then dried for 24 h at 80°C for determinations of the dry weigh (Wd). Relative water content (RWC) was calculated by the following formula: RWC = (Wf – Wd)/(WS – Wd) x 100% as given by [19]. |
| D. Enzymatic Oxidative Defense Mechanism (SOD, CAT, APX and GR) The method of [20] was followed with slight modification for estimation of Superoxide dismutase (SOD activity: E.C 1.15.1.1) activity. Fresh algal mass (50 mg) was homogenized in 2 mL of extraction mixture [1.5 mL Na-phosphate buffers (0.5 M, pH 7.3), 0.1 mL of EDTA (3 mM), 1g PVP (1%), 1 mL Triton X 100 (1%)] and centrifuged at 11269 g at 4°C. SOD activity in the supernatant was assayed by its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT). The assay mixture, (consisting of reaction buffer containing 1.5 mL Na- phosphate buffer (0.1M, pH 7.5), 1g PVP 1% , 0.2 mL L-methionine, 0.1mL enzymes extract with equal amount of 1.5 mL NaHCO3, 0.1 mL NBT solution (2.25mM), 0.1 mL EDTA (3 mM), 0.1 mL riboflavin (60 μM) and 1.0 mL DDW was incubated under 15 W inflorescent lamp at 28°C. 50% reduction of NBT was considered as one unit of enzyme activity. Catalase (CAT activity E.C 1.11.1.6) activity was determined by the method of [21]. Algal biomass (50 mg) was homogenized in 3 mL extraction buffer [1.5 mL Na-phosphate buffers (0.5 M, pH 7.3), 0.1 mL EDTA (3 mM), 1g PVP (1%), 1mL Triton X 100 (1%)] and centrifuged at 7826 g for 20 min at 4°C. CAT activity in supernatant was determined by monitoring the disappearance of H2O2, measuring a decrease in absorbance at 240 nm. Reaction was run in a final volume of 1mL of reaction buffer containing [0.1 mL Na-phosphate buffers (0.5 M) pH 7.3, 0.1 mL EDTA (3 mM), 0.2 mL of enzyme extract and 0.6 mL H2O2 (3 mM)] for 3min. CAT activity was calculated by using coefficient of absorbance of 0.036mM-1 cm.-1. One unit of enzyme determines the amount necessary to decompose 1μmol of H2O2 per min. Ascorbate Peroxidase (APX activity: E.C 1.11.1.11) activity was estimated by the method of [22]. Algal biomass (50 mg) was homogenized in 3 ml extraction buffer [1.5 mL Na-phosphate buffers (0.5 M, pH 7.3), 0.1 mL EDTA (3 mM), 1g PVP (1%), 1mL Triton X 100 (1% )], and centrifuged at 7826 g for 20 min at 4°C. APX activity was determined in supernatant by the decrease in absorbance of ascorbate at 290 nm, due to its enzymatic break down. Reaction was run in a final volume of One ml of reaction buffer containing 0.1 mL ascorbate (0.5 mM), 0.6 mL H2O2 (0.1 mM), 0.1 mL EDTA (0.1 mM) and 0.2 mL of extract containing enzyme. The reaction was run for 3 min at 25°C. APX activity was calculated by using coefficient of absorbance 2.8 mM-1 cm.-1 One unit of enzyme determines the amount necessary to decompose 1 μmol of ascorbate per min. Glutathione reductase (GR: E.C 1.6.4.2) activity was determined by the method of [23] modified by [24]. Algal biomass (50 mg) was homogenized in 2 ml extraction buffer [1.5 mL Na-phosphate buffers (0.5 M, pH 7.3), 0.1 mL of EDTA (3 mM), 1g PVP (1%), 1 mL Triton X 100 (1%)], and centrifuged at 7826 g for 10 min at 4°C. The supernatant was immediately assayed for GR activity through glutathione- dependent oxidation of NADPH at 340 nm. One mL reaction mixture containing 0.3 mL NADPH (0.2 mM), 0.5 mL GSSG (0.5 mM) and 0.2 mL enzyme extract were kept for 5 min at 25 °C. Correction was made for any GSSG oxidation in the absence of NADPH. The activity was calculated by using coefficient of absorbance 6.2 mM-1 cm.-1 One unit of enzyme determines the amount necessary to decompose 1μmol of NADPH per min. E. Non-Enzymatic Oxidative Defence Mechanism (GSH, GSSG, GSH+GSSG and proline) Reduced glutathione (GSH), oxidized glutathione (GSSG) and total glutathione (GSH+GSSG) were determined by the glutathione recycling method of [25]. Algal biomass (50 mg) was homogenized in 2 mL sulphosalicylic acid (5%) at 4°C. The homogenate was centrifuged at 7826 g for 10 min. To a 0.5 mL supernatant, 0.6 mL of reaction buffer (2 mL Naphosphate buffer (0.1M, pH 7.0), 0.1 mL EDTA 1mM and 0.04 ml of 0.15% 5,5 dithiobis-2- nitrobenzoic acid (DTNB) were added and after 2 minutes the absorbance was read at 412 nm. To the same, 0.04 mL NADPH (0.4%) and 0.002 mL of Glutathione reductase (GR; 50mg enzyme unit) were added and reaction was run for 30 min at 25°C. The absorbance was again read at 412 nm to determine the total glutathione. F. Proline Estimation Proline content in algal biomass was estimated by the method of [26]. 250 mg wet biomass was homogenized in 10 ml 3% sulphosalicylic acid and centrifuged at 7826 g for 10 min. To 2.0 ml the filtrate / proline solution added 2 ml glacial acetic acid and 2 mL ninhydrin solution and placed in a boiling water bath for about 1 hour. Reaction was stopped in an ice bath and then 4 ml toluene was added to each sample. The samples were shaken on a vortex and then were kept standing for 30- 60 seconds to allow the organic layer to separate out. The absorbance was read at ïÃÂì520 on a UV-Vis spectrophotometer (Modle DU-640B, Beckman, CA, USA). The proline content was obtained from the standard curve. |
III. RESULT & DISCUSSION |
| Growth is a good indicator of the action of noxious compounds in susceptible microorganisms, and reflects the metabolism of the cell. Exogenous addition of varying concentration of sodium arsenate (0-150 mM) for different duration (24 hrs, 32 hrs and 72hrs) showed significant reduction in growth of Cyanobacterium H.fontinalis-339. Maximum reduction was observed at 150 mM of Sodium Arsenate. Sublethal concentration was found at about 100mM sodium arsenate when exposure duration was 24 hrs, 32 hrs and 72hrs [Fig 1(a)]. At 70mM sodium arsenate 50% decline in growth was found [Fig 1(b)]. |
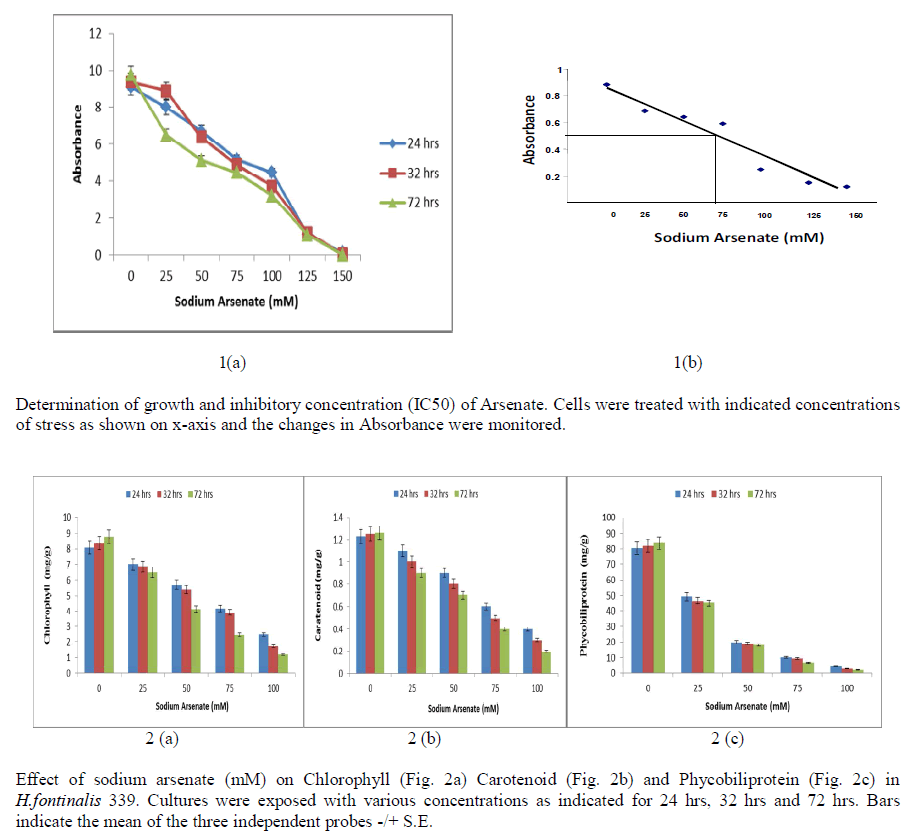 |
| The results were highly indicative of their inhibitory effects on photosynthetic activities of the cells in a time dependent manner. These results find support from the work of [28] who has reported inhibition of chlorophyll and phycocyanin in Anabaena doliolum by As (III) and As (V). The reduction of chl-content may be due to the inhibition of chlorophyll biosynthesis by arsenic mediated inhibition of δ-aminolevulinic acid dehydrogenase [29] or accelerated degradation of the pigment [30]. The decline in pigment content may be due to the lysis of cell wall and disruption of thylakoid membrane as reported in Anabaena flosaquae in presence of arsenic. The depletion of algal pigments seems to be result of photooxidation induced by be inability of chlorophyll to dissipate its absorbed excitation energy due to inhibition of electron transport. The pigment degradation may be due to binding of the metal onto the thylakoid membranes, which eventually leads to membrane damage and impedes photosynthetic activities. In present study significant increase in ROS (lipid peroxidation and MDA) was also observed with increasing arsenate concentration and exposure duration (Fig. 3a). The 100mM sodium arsenate exposure for 72 hrs resulted in maximum MDA increase (200%). Sodium arsenate also induced a dose exposure dependent increase in electrolyte leakage (Fig. 3b). |
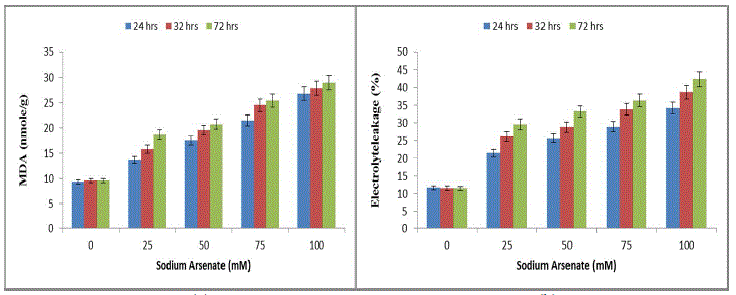 |
| Effect of sodium arsenate (mM) on MDA content (Fig. 3a) Electrolyte leakage (Fig. 3b) in Hapalosiphon fontanilis-399. Cultures were exposed with various concentrations as indicated for 24 hrs, 32 hrs and 72 hrs. Bars indicate the mean of the three independent probes -/+ S.E. (Fig. 4) 24 hr exposure resulted in 194% increase in membrane leakage after 72 hrs that reached to 269%. The data of lipid peroxidation and membrane leakage indicated Sodium arsenate induced oxidative damage that may be attributed to its known role in uncoupling the oxidative phosphorylation, thereby inhibiting ATP synthesis and disrupting the ΔpH thus leading to leakage of electrons [31]. Not only has this ROS increase also resulted in decrease of growth by inactivation of enzymes. Enhanced generation of reactive oxygen species serve as signalling molecule in acclimation and defence responses [32]. The enzyme SOD is in the first line of the enzymatic antioxidant defence system. It converts O. 2, to H2O2 and O2 [33]. Total SOD activity was significantly (P< 0.05) increased in H. fontinalis-339 in presence of Sodium arsenate. SOD increase was observed with respect to time (268%) at 24 hrs and after that at 72 hrs maximum increase was observed (361%) was detected in 100mM sodium arsenate (Fig. 4a). Under arsenic stress enhanced activity of SOD was recently observed in Phormidium laminosum [11]. Enhanced SOD activity has already been reported due to Cu, Cd and Pb in cyanobacterium like Spirulina platensis, Hapalosiphon fontinalis-339 [34]-[35]. Catalase is an important enzyme in the removal of H2O2. In our study at low levels of Sodium arsenate (25mM) CAT activity was higher (120%) than control showing protective role but at higher concentration of Sodium arsenate its activity decreased (Fig. 4b). Decline observed at higher concentration of arsenic might be attributed to the inactivation of enzyme by ROS or change in assembly of enzyme subunit [36-37]. APX is an important enzyme of ascorbate-glutathione cycle and utilize ascorbate (AsA) as its specific electron donor to reduce H2O2 to water with the concomitant generation of monodehydroascorbate (MDHA) that spontaneously disproportionate to AsA and dehydroascorbate (DHA). APX activity significantly (P<0.05) showed a concentration and time dependent increase. 100mM arsenate exposure for 24 to 72 hrs resulted in 156% upto 188% increase in APX respectively (Fig. 4c). But decrease activity of APX is reported in laboratory grown Phormidium in presence of Sodium arsenate [11]. Glutathione Reductase (GR) is involved in defence against oxidative stress, whereas reduced glutathione (GSH) plays an important role within the cell system, including participation in the ascorbate-glutathione cycle, maintenance of the sulphydryl groups of cysteine in a reduced form, storage of reduced sulphur and a substrate for Glutathione sulphur transferase. In the present study, GR activity significantly (P<0.05) increased in a concentration and time dependent manner (Fig .4d). Maximum (213%) increase was observed at 100mM. |
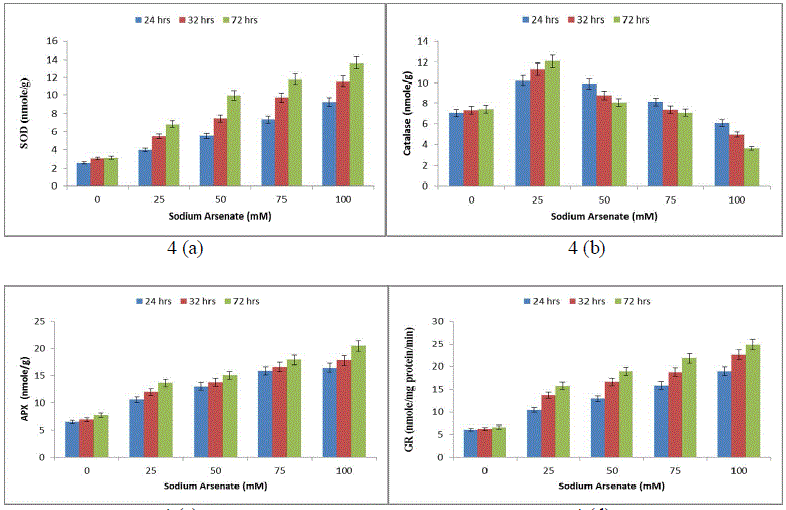 |
| Effect of sodium arsenate (mM) on Superoxide dismutase (SOD) (Fig. 4a) Catalase (CAT) (Fig. 4b) Ascorbate peroxidase (APX) (Fig. 4c) Glutathione reductase (GR) (Fig. 4d) in Hapalosiphon fontanilis-399. Cultures were exposed with various concentrations as indicated for 24 hrs, 32 hrs and 72 hrs. Bars indicate the mean of the three independent probes -/+ S.E. The non-enzymic antioxidant reduced glutathione (GSH), showed (52%) 24 hrs and 80% (72 hrs) decrease with respect to time as well as dose (Fig. 5a), while oxidized glutathione (GSSG) and total glutathione (GSSG+GSH) increased rapidly and the increase was 114% and 181% in a dose and time dependent manner respectively (Fig. 5b & c). The reduction of Reduced glutathione might be due to utilization ascorbate regeneration as electron donor which is required by APX, regulates the protein thiol-disulfide exchange reactions [38], increases stress tolerance, and seems to be an important signal molecule [39]. A decline in the GSH pool of the cell can be attributed to the utilization of GSH in some metal binding protein e.g. Metallothonine and phytochelatin synthesis [40]. The increase in total glutathione (GSSG+GSH) in our study might be attributed to the fact that the synthesis of reduced glutathione (GSH) is a demand driven process; it is being used for Phytochelatin formation that triggered increased sulphur uptake and its own synthesis and is in agreement with Stichococcus bacillaris under arsenic [41]. |
| An increasing trend in proline concentration was observed from 24 hrs to 72 hrs compared to the control. The proline increase was 180% upto 340% in 100 mM Sodium arsenate (Fig. 5d) indicating that proline also plays an important role in arsenic resistance stress in H. fontinalis-339. |
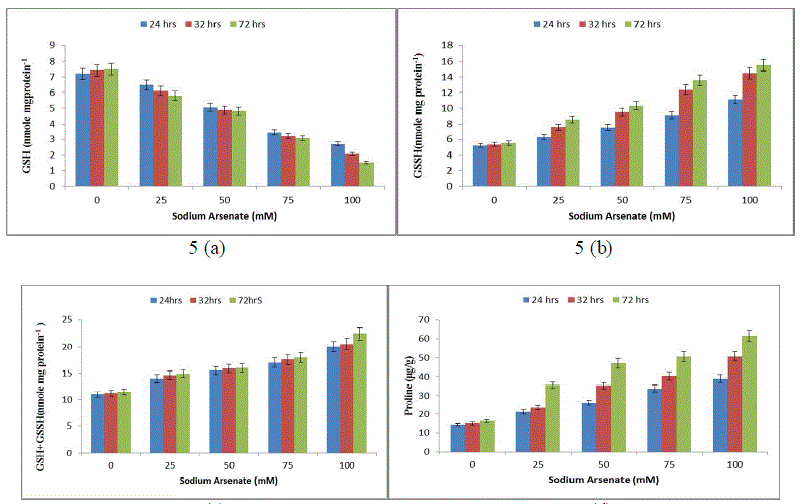 |
| Effect of sodium arsenate (mM) on Reduced glutathione (GSH) (Fig. 5a) Oxidized glutathione (GSSH) (Fig. 5b) Total glutathione (GSH + GSSH) (Fig. 5c) Proline (Fig. 5d) in Hapalosiphon fontanilis-399. Cultures were exposed with various concentrations as indicated for 24 hrs, 32 hrs and 72 hrs. Bars indicate the mean of the three independent probes -/+ S.E. An almost similar effect was earlier observed in Oryza sativa, under a condition of As2O3 stress [42]. Proline acts as signalling molecule to activate multiple responses that are part of the adaptation process [43]. Elevated levels of proline have been shown to play a protective role by preventing enzyme inactivation [44]-[45]. Proline accumulates rapidly and more frequently than any other amino acid under unfavourable environmental conditions [46], especially in drought and salt stress. It could be involved in stress resistance mechanisms by acting as an Osmoprotectant, thereby facilitating Osmoregulation and stabilization of cytosolic acidity [44]. Involvement of proline in cyanobacterial defence mechanism has been reported for the first time from our lab in presence of pesticide (endosulfan, alphamethrin and deltamethrin) and heavy metal in Nostoc muscurum, Anabaena variabilis, Aulosira fertilissima, Spirulina plantensis-S5 and Westiellopsis prolific-Janet strain-NCCU331 [47], [35]-[48]. |
IV. CONCLUSION |
| In the present study, the toxic effect of sodium arsenate appears to be related to the production of reactive oxygen species and the resulting unbalanced cellular redox status. Cyanobacteria H.fontinalis responds to sodium arsenate by alteration in activities of several antioxidant enzymes such as superoxide dismutase, glutathione reductase, ascorbate peroxidase, and non-enzymatic substances as carotenoids, glutathione and proline. |
ACKNOWLEDGEMENTS |
| This work was supported by Council of Scientific and Industrial Research, New Delhi, Government of India as Research Associatship to Miss Sunaina Zutshi (Ph.D). |
References |
|