ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Dr.Rita Gharde1 and Mrs.Manisha G. Bhave2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Liquid crystals are characterized by high molecular orientation order in their mesomorphic states. The mixture of liquid crystals influences the molecular orientation of liquid crystal compound consequently affecting their physical properties. In this paper, the main focus is on the study of the mixtures of Nematic liquid crystal and Cholesteric liquid crystal in different proportions. We have used Differential scanning calorimetry (DSC) technique to measure the Phase transition temperatures. Fourier Transform Infrared Spectroscopy (FTIR) and Ultraviolet–Visible (UV) spectroscopy are used to analyze the relative concentration of liquid crystals in the mixtures and their spatial distribution.The values of pitch for Cholesteric liquid crystal and the mixtures were calculated from ordinary refractive indices. Because of its low phase transition temperatures and less requirement of energy, the mixture of Cholesteric chloride with Cholesteryl oleate in 30%+70% proportion holds promise in the optical as well as non-optical applications of liquid crystals.
Keywords |
| Liquid Crystal (LC), Differential scanning calorimetry (DSC), Fourier Transform Infrared Spectroscopy (FTIR), Ultraviolet Visible (UV) Spectroscopy, Pitch. |
INTRODUCTION |
| Liquid crystals have become a consumer product with many everyday applications. A liquid crystal is a mesophase which has partially or completely lost the long range order of ordinary crystals, but still possesses one or more dimensional long range orientational order of certain anisometric units. [1], [2],[3] The mixture of liquid crystals bring the melting point down which helps or gives wide applicability of its use in various applications. These mixtures show phase transition temperatures and other physical properties which are different from their constituents.[4],[5]. In the present study the main focus is on the miscibility of Nematic liquid crystal and Cholestaric liquid crystal. Arnold,Sackman and Demus developed the miscibility rules which can be summarized as follows. [6], [7], [8], [9]. i) If two LCs are miscible, they are isomorphic and therefore belong to the same type of mesophase, ii) If two LCs are isomorphic, they need not be necessarily miscible. |
| When two compounds are isomorphic within a certain mesophse, both their thermal transition temperatures and corresponding thermodynamic parameters exhibit continuous dependence on their components. This means that both the components of mixtures behave like an ideal solution. Therefore, by knowing the transition temperatures and thermodynamic parameters of the parent compounds, we can apply the equations of Schroeder and Van Lar(equation 1)to predict the phase diagram of the mixtures. |
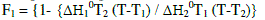 |
RELATED WORK |
| In studying liquids crystals, we often want to expand the range of certain phases so the phase is more stable.[11] We also to alter the physical properties such as viscosity. An easy way to do this is to dope the liquid crystal with another liquid crystal.[12]The mixtures help in realignment of the liquid crystal molecules which can have a large effect on the electrooptical properties of a liquid crystal.[13],[14] .The previous researchers studied various properties of the liquid crystal mixtures using different techniques. V.S,Chandel,A.K.Singh,S.Manohar,J.P.Shukla and R.Manoher[15] studied phase transitions in binary mixture of Cholesteric liquid crystals. Their main focus is on different phases of liquid crystal mixtures. They not only observed new phases in the mixtures but also found higher thermal stability in the mixtures. Authors [16] measured dielectric, refractive, elastic and viscous properties of the HBLCMs.The mixtures measured high optical and dielectric anisotropies and relatively low rotational viscosities. This has applications in different kinds of liquid crystal electro-optical devices with low response times, operating in visible and near infrared regions. PK Mukherjee[17] studied the mixtures of HBAB {p-[(p-hexyloxy-benzylidene)-amino] benzonitrile} and CBOOA [N-p-cyanibenzylidene-p-n-octyloxyaniline].The mixtures exhibited a two phase region where both smectic-A and nematic phase coexists. is discussed by means of Landau formalism. The problem of the first or second order nature of the nematic-smectic-A phase transition is explored. General Landau theory for coupled orientational, translational order parameters and concentration is developed by the author to discuss the reason for this two phase region. Authors [18] formulated two exemplary high birefringence and low clearing temperature Liquid crystal mixtures, using the laterally substituted isothiocyannato tolane compounds. They observed decrease in the melting point (below 200C) in the mixtures. The temperature derivative of the mixtures was enhanced in the mixtures. |
LIST OF CHEMICALS AND SAMPLE PREPARATION |
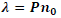 |
EXPERIMENTAL TECHNIQUES |
| The essential tools used in present study are Differential scanning calorimetry (DSC), Fourier Transform Infrared Spectroscopy (FTIR), Ultraviolet Visible (UV) Spectroscopy and ordinary refractive indices using multiwavelength Abbe refractometer |
| 1 Differential scanning calorimetry (DSC) |
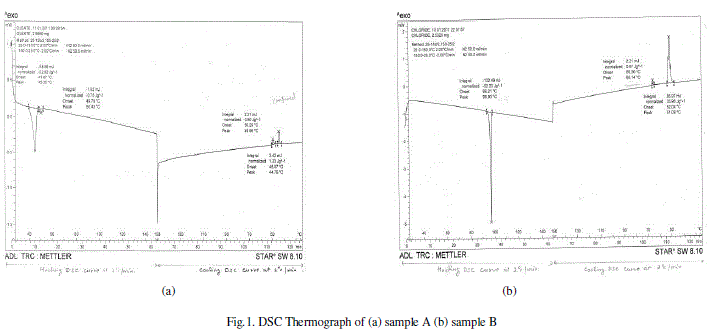
| DSC is a thermoanlytical technique in which the difference in the amount of heat required to increase the temperature of the sample and reference is measured as a function of temperature. Both the sample and reference are maintained nearly at the same temperature throughout the experiment. The temperature of the sample holder increases linearly as a function of time. Even small changes in thermodynamic quantities at transitions between mesophases can be detected by DSC.The result of a DSC experiment is a curve of heat flux versus temperature or versus time. Most liquid crystal to liquid crystal transitions are discontinuous. Such transitions are revealed by larger peaks. |
| 2 Fourier Transform Infrared Spectroscopy (FTIR) |
| FTIR is a powerful tool for identifying types of chemical bonds in a molecule by producing an infrared absorption spectrum that is like a molecular fingerprint. The FTIR spectroscopy detects the vibration characteristics of chemical functional groups in the sample.[10] When infrared light interacts with matter, chemical bonds will stretch, contract and bend. The chemical functional group absorbs IR radiation in a specific wave number range. It is a chemical analytical technique that measures the infrared intensity vs wavelength of light. The IR is divided into three regions i.e. far infrared ( 4 – 400 cm-1 ) , mid infrared ( 400 – 4000 cm-1 ) and near infrared (4000 – 14000 cm-1 ) It can be applied to the analysis of types of chemical bonds i.e. functional groups.IR spectroscopy works because chemical bonds have specific frequencies at which they vibrate corresponding to energy levels. From the recorded FTIR spectra various functional groups of different samples can be identified. |
| 3 Ultraviolet Visible (UV) Spectroscopy |
| UV spectroscopy is an accurate and powerful procedure to analyze a substance. It measures the absorption, transmission and emission of ultraviolet and visible light by matter. Absorption of ultraviolet or visible light causes electron to move from lower to higher energy levels. Because the spectrum of an atom or molecule depends on its electron density level, it is useful for identifying unknown substances. |
| 4 Determination of pitch from RI |
| Liquid crystals are birefringent in nature. When the light enters liquid crystals it splits into an ordinary ray and an extraordinary ray. The ordinary ray moves faster than the extraordinary ray. The cholesteric liquid crystals consist of thin birefringent layers normal to the optic axis and each one is turned through a small angle with respect to its neighbours.Because of this turn each linear component of the light experiences a change in refractive index while passing from one layer to the next .The changes will be an increase for the fast and a decrease for the slow component. By measuring the refractive index of ordinary ray (no) for the given wavelength of light (λ), pitch (P) of the Cholesteric liquid crystal and the mixtures can be calculated using the relation, |
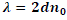 |
RESULTS AND DISCUSSION |
 |
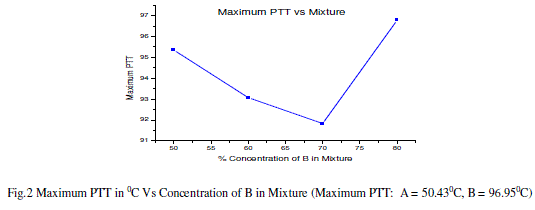 |
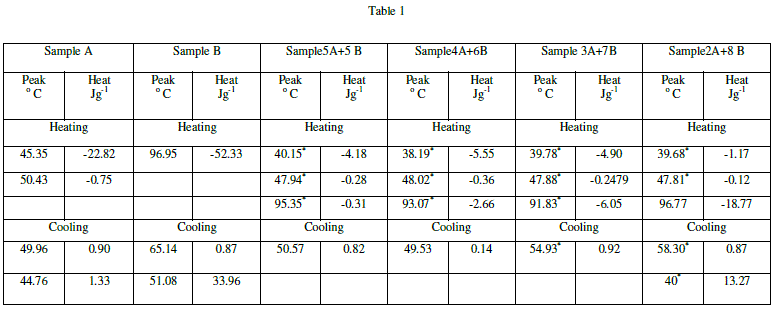 |
| From Table 2 we can conclude that though the wavelengths are common, the intensities of absorption for the mixtures are different. This is because the sample molecules selectively absorb radiation of specific wavelengths which causes the change in the dipole moment. Consequently, the vibrational energy levels of sample molecules transfer from ground state to excited state. The frequency of the absorption peak is determined by the vibrational energy gap. The number of absorption peaks is related to the number of vibrational freedom of the molecule. The intensity of absorption peaks is related to the change of dipole moment and the possibility of the transition of energy levels. So the less value of the intensity of absorption indicates the stability in the structure. The mixture 3A+7B has less value of the intensity of absorption. This indicates that the mixture3A+7B has more stability amongst all the mixtures of A and B. |
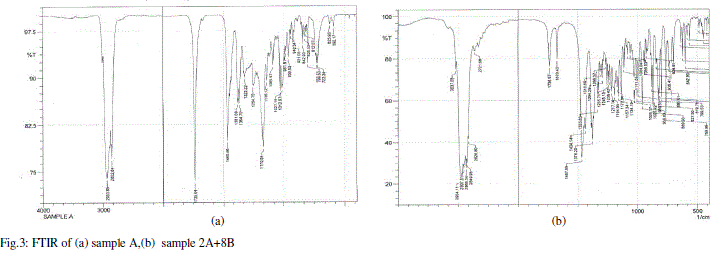
| When a liquid crystal molecule absorbs IR, it’s chemical bonds vibrate, the bonds can stretch, contract and bend. From the FTIR graphs for sample A, B, 5A+5B, 4A+6B and 3A+7B,2A+8B the values of the stretching in the wavelengths are measured and indicated in table 3.The corresponding bonds are also given in the same table. |
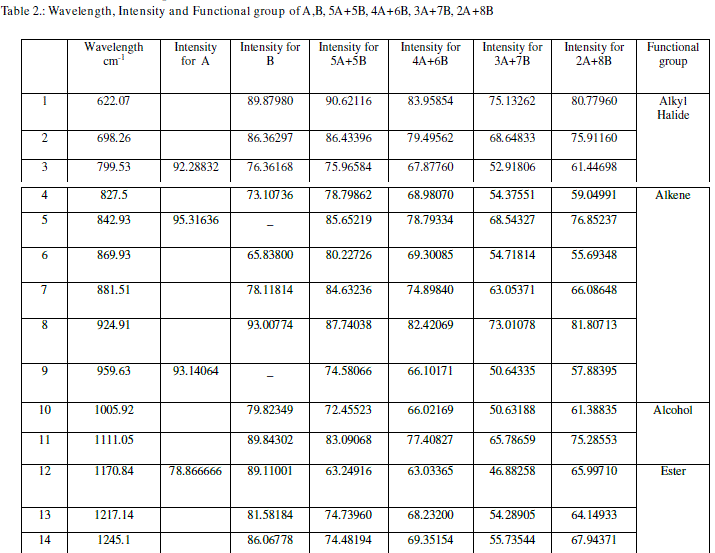 |
| From table 3 we can conclude that the bond present in mixtures 5A+5B, 4A+6B, 3A+7B and 2A+8B is C=C. |
 |
 |
 |
| The calculated values of pitch for all the mixtures match with the value of the pitch of cholesteric liquid crystal (sample B). For all the wavelengths, the value of pitch is minimum for the mixture 3A+7B.Also; the distance between the two successive layers of the mixture 3A+7B is minimum amongst all the values. |
CONCLUSION |
| We studied the miscibility of Nematic liquid crystal with Cholesteric liquid crystal, with increasing concentration of Cholesteryl Chloride in the mixtures. The transition temperatures obtained by Differential scanning calorimetry (DSC) were found to change depending on the proportion of the constituents. Lower transition temperatures were obtained when Cholesteric chloride is mixed with Cholesteryl Oleate in 30%+70% proportion indicating stabilizing effect of pitch of Cholesteric Chloride in the mixture. The Fourier Transform Infra-red Spectroscopy (FTIR) and Ultraviolet – Visible (UV) Spectroscopy techniques confirmed the miscibility of the two liquid crystals. The stability of the mixture 3A+7B was also confirmed by these techniques. The pitch and the spacing between thin birefringent layers is reduced in the mixture 3A+7B.So we can conclude that among all four mixtures studied by us, the mixture of Cholesteryl Oleate and Cholesteryl Chloride in 30%+70% proportion is more stable. Because of its low phase transition temperatures and less requirement of energy, the mixture of Cholesteric chloride with Cholesteryl oleate in 30%+70% proportion holds promise in the optical as well as non-optical applications of liquid crystals. |
ACKNOWLEDGEMENT |
| I would like to thank Dr.Anuradha Mishra, Head, Department of the Physics, University of Mumbai and Dr.G.V,Rao ,I/C Principal, Siddharth College for their support. |
References |
|