ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| +Rajesh Kumar Dubey1,Priyanka Dixit2, Sunita Arya3 Director General, PERI, M-2/196, Sector-H, Aashiana, Lucknow-226012,UP, India1 Department of Biotechnology, SVU Gajraula, Amroha UP, India1 Assistant Professor, MGIP, Lucknow, UP, India2 Assistant Professor, DGPG College, Kanpur,UP, India3 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Until recently, pharmaceuticals used for the treatment of diseases have been based largely on the production of relatively small organic molecules synthesized by microbes or by organic chemistry. These include most antibiotics, analgesics, hormones, and other pharmaceuticals. Increasingly, attention has focused on larger and more complex protein molecules as therapeutic agents. This publication describes the types of biologics produced in plants and the plant based organic therapeutic agent's production systems in use.
Keywords |
| Antecedent, Antibiotics; Anticancer;Antiparasitic; Antileishmanial;Antifungal Analgesics; Flavonoids; Hormones; Pharmaceuticals |
INTRODUCTION |
| Naturally occurring pharmaceutical and chemical significance of these compounds offer interesting possibilities in exploring their more pharmacological and biocidal potentials. One of the main objectives of organic and medicinal chemistry is the design, synthesis and production of molecules having value as human therapeutic agents [1]. Flavonoids comprise a widespread group of more than 400 higher plant secondary metabolites. Flavonoids are structurally derived from parent substance flavone. Many flavonoids are easily recognized as water soluble flower pigments in most flowering plants. According to their color, Flavonoids pigments have been classified into two groups:(a) The red-blue anthocyanin's and the yellow anthoxanthins,(b)Aurones are a class of flavonoids called anthochlor pigments [2]. Flavonoids are a diverse group of natural products that play important roles in plant growth and development and in defenses against microorganisms and pests. Apart from their physiological roles in plants, flavonoids are important antioxidants in the human diet that can scavenge free radicals. Biosynthetically, flavonoids are derived from chalcone precursors that in turn are derived from the condensation of p-coumaroyl CoA and three malonylCoAs by chalcone synthase [3].Aurones are a class of flavonoids found in fruits and flowers where they function as phytoalexins against infections and contribute to the yellow pigmentation of plant parts [4]. Aurones are obtained from chalcones by aurone synthase as well as through the biosynthesis of other flavonoids. Aurones have been reported to possess insect antifeedent, anticancer, ant parasitic, antileishmanial and antifungal activity. Aurones can be used as potential cancer chemotherapy agents and as inhibitors of an enzyme involved in the metabolism of thyroid hormones. They have also been reported to be ant proliferative agents, tyrosinase inhibitors, antimicrobial agents and as potentially useful imaging agents for detecting β- amyloid plaques in Alzheimer’s disease [5]. |
| Chromones (1-benzopyran-4-one) are a group of naturally and widely distributed compounds which are ubiquitous in nature, especially in the plant kingdom. They are oxygen-containing heterocyclic compounds with a benzo-annelated gpyrone ring. Molecules containing the chromone structure (such as flavonoids and chromones) receive considerable attention in the literatures recently, mainly due to their biological and physiological activities including ant mycobacterial, antifungal, anticonvulsant, antimicrobial, mushroom tyrosinase inhibition activities, intermediates to many products of fine chemical industries [6]. Chromones having heterocyclic substituents at 2 and 3 positions have been reported to possess antiallergic activity, muscular relaxation effect and anti-microbial activity [7]. Literature survey clearly indicates that flavones (chromones) and aurones have been studied largely for their therapeutic potential. In this review, we report the recent advances made on therapeutic potential of aurones and chromones in different geographical areas. The synthesis, structureactivity relationships, the importance of the substitution pattern shall be discussed [8]. |
II. LITERATURE REVIEW |
| Chandra et al. (2010) carried out the preparation of some oxadiazolylpyrazol-5-ones with the objective of disovering potent anti-inflammatory agents. All the compounds exhibited anti-inflammatory and analgesic activities at the dose 50mg/kg. Thecompound 1-(2’,4’-chloroacridine-9’-yl)-3-(5’-pyridine-4-yl)-(1,3,4-oxadiazol-2-yl-thiomethyl)-pyrazol-5-one I showed better anti-inflammatory and analgesic activities at doses of 25, 50 and 100mg/kg body weight [9].A series of 3,5- diaryl-isoxazoline/isoxazole linked pyrrolo[2,1-c][1,4]benzodiazepine II conjugates were synthesized by Kamal et al. (2010). These conjugates showed potent anticancer activity. Some of these conjugates with promising anticancer activity were further investigated on the cell cycle distribution. Moreover, these conjugates exhibited G0/G1 arrest, enhancement in the levels of p53 protein as well as mitochondrial-mediated intrinsic pathway, leading to release of cytochrome c, activation of caspase-3, and subsequent apoptotic cell death [10]. Conti et al. (2010) carried out the synthesis of a series of novel isoxazole-based histone deacetylase (HDAC) inhibitors, structurally related to SAHA (suberoylanilidehydroxamic acid) as first-in-class HDAC inhibitors, for the treatment of cutaneous T-cell lymphoma (CTCL). The new compounds were subjected to biological evaluation to identify the molecules endowed with HDAC inhibitory activity. Compound III2demonstrated to have a dose dependent moderatebiological activity. However, the inhibitory activity was lower than that measured for thereference compound SAHA, especially when measured at low concentrations [11]. |
| A series of potential antioxidant and antibacterial N’-arylmethylidene-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3- c][1,2]benzothiazin-2(4H)-yl)acetohydrazides IV were synthesized by Ahmad et al. (2010) in ultrasonic mediated conditions. The reaction was completed in excellent yields of upto 86% [12].Franchiniet al. (2010) synthesized a series of novel pyrazolesV and evaluated them as potential inhibitors against U87MG glioma cell lines. Most of the pyrazoles showed inhibitory activity at a concentration higher than 150μM. Three of these compounds showed promising activity on cell growth [13].Abele et al. (2009) synthesized 3-oxime of 2H-[1,4]benzoxazine-2,3(4H)-dioneVI by the cyclization of oamino- phenol in the presence of the Z-isomer of ethyl chloro(hydroxyimino)acetate and triethylamine in diethyl ether [14]. A series of 2-pyrazolines VII were synthesized by condensing chalcones with 4-hydrazinonbenzenesulfonamide hydrochloride by Rathishet al. (2009). The compounds were screened for their anti-inflammatory activity in carrageenaninduced rat paw edema model. Compounds showed COX-1 and COX-2 inhibitory activity at the dose of 20 mg/kg [15].Khodeet al. (2009) synthesized a series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines VIII as antiinflammatory agents and found that 4- chloro and 3- methoxy groups in coumarinylpyrazolines give rise to an increased anti-inflammatory activity [16]. Raiet al. (2009)carried out the synthesis of a series of novel 2-[1-(5-chloro-2-methoxy-phenyl)-5-methyl-1H-pyrazol-4-yl]- 5-(substituted-phenyl)-[1,3,4]oxadiazoles IX, by cyclization of substituted-benzoic acid with N’-[1-(5-chloro-2-methoxyphenyl)- 5-methyl-1H-pyrazole-4-carbonyl]-hydrazide using phosphorousoxychloride at 120°C. All the compounds exhibited promising bactericidal activities [17].Abele et al. (2009) synthesized 1,4-thiazine oximes by the cyclization of 2- amino-ethanethiols or 2-aminothiophenol with ethyl 2-chloro-2-(hydroxy-imino)acetate, ethyl nitroacetate or nitroacetone in alkaline medium. By this approach, 2-hydroxyiminotetrahydro-1,4-thiazin-3-ones X were obtained in yields of up to 83% [18]. Gummudavellyet al. (2009) synthesized novel isoxazole derivatives XI and screened them for anti-microbial and antiinflammatory activities. It was found that compounds showed comparable activities as that of the standard drug, ibuprofen [19].Karabasanagoudaet al. (2009) synthesized pyrazolineXII and isooxazoleXIII derivativesas anti-inflammatory agents and found that the chloro and methoxy substituted phenyl ring at 5- position of pyrazoline and isoxazole showed potent anti-inflammatory activity [20].Reaction of chalcones with hydroxylamine has been investigated by Voskieneet al. (2009). By this approach isoxazole derivatives XIV were synthesized in good yields [21]. A series of coumarin substituted pyrazolesXV were prepared by Ahmad et al. (2009). Compounds were screened for their anti-inflammatory activity at a dose of 20mg/kg. The compounds inhibited formalin induced hind paw edema and also significantly suppressed the formation of granuloma tissue in cotton pellet induced chronic model of inflammation [22].Popov et al. (2009) prepared novel benzo[e][2,1]thiazine derivatives and found that the 4-chlorobenzo[e][2,1]thiazinesXVI were obtained in yield of up to (95-99%) via convenient protocols involving oxidation and reduction reactions of chloroaldehydes and chloronitriles. The structures of all the products were established from the NMR spectra, elemental analysis and mass spectra [23]. |
III. CHEMISTRY OF AURONE & CHROMONE |
| Chromone (1, 4-benzopyrone) is a derivative of benzopyran with a substituted keto group on the pyran ring. Derivatives of chromone are collectively known as chromones. Most, though not all, chromones are also phenylpropanoids [24]. Aurone is a heterocyclic chemical compound that contains a benzofuran element associated with a benzylidene linked in position 2. In aurone,achalcone group is closed into a 5-membered ring instead of the 6-membered ring more typical of flavonoids [25]. Some most important plant based is as follows: 1. Antirrhinum majus: The yellow coloration of snapdragon (Antirrhinum majus) flowers is mainly provided by the 6-glucosides of aureusidin and bracteatin. However, the biochemical mechanism of aurone biosynthesis is not well understood. In this study, we have identified aurone-biosynthesizing activity in the extracts of yellow snapdragon flowers. Incubation of 2′, 4′, 6′, 4- tetrahydroxychalcone (THC) with an enzyme preparation in the presence of H2O2 caused the enzymatic formation of a single product, aureusidin, without the formation of a previously proposed 2-(α-hydroxybenzyl) coumaranone intermediate. The formation of aureusidin from THC was specifically observed with yellow flowers as well as aurone-accumulating flowers of other colors. The pH optimum for the enzymatic formation of aureusidin was around 5.4. Stoichiometric studies showed that one mole of aureusidin formation were accompanied by the consumption of one mole of oxygen with no detectable consumption of H2O2, which may work as an enzyme activator. The oxidative formation of aureusidin from THC could be explained in terms of the action of a single enzyme, an internal monooxygenase catalyzing the 3-hydroxylation and oxidative cyclization of THC. Incubation of 2′, 4′, 6′, 3, 4-pentahydroxychalcone (PHC) with an enzyme yielded both aureusidin and bracteatin at an approximate molar ratio of 6:1. In this case, H2O2 was not required for enzyme activity but rather inhibited the reaction. The 4′-glucosides of THC and PHC could also act as substrates for the formation of the 6-glucosides of aurones. These results suggest that aureusidin can be produced from either THC or PHC, whereas bracteatin is not produced through the hydroxylation of aureusidin but arise solely from PHC [26]. |
 |
| 2. Medicagotruncatula: |
| An integrated approach utilizing HPLC–UV–ESI–MS and GC–MS was used for the large-scale and systematic identification of polyphenols in Medicago truncatula root and cell culture. Under optimized conditions, we were able to simultaneously quantify and identify 35 polyphenols including 26 isoflavones, 3 flavones, 2 flavanones, 2 aurones and a chalcone. All identifications were based upon UV spectra, mass spectral characteristics of protonated molecules, tandem mass spectral data, mass measurements obtained using a quadrupole time-of-flight mass spectrometer (QtofMS), and confirmed through the co-characterization of authentic compounds. In specific instances where the stereochemistry of sugar conjugates was uncertain, subsequent enzymatic hydrolysis of the conjugate followed by GC–MS was used to assign the sugar stereochemical configuration. Comparative metabolic profiling of Medicago truncatula root and cell cultures was then performed and revealed significant differences in the isoflavonoid composition of these two tissues [27]. |
| 3. Glycyrrhizauralensis: |
| Y.B. Ryuet. al.,isolated 18 polyphenols with neuraminidase inhibitory activity from methanol extracts of the roots of Glycyrrhiza uralensis. Individual compounds were evaluated for their neuraminidase inhibitory activity. These polyphenols consisted of four chalcones, nine flavonoids, four coumarins, and one phenylbenzofuran [28]. |
| 4. Caraganaconferta: |
| Confertins A (1) and B (2), new 3-C-carboxylated flavones, have been isolated from the ethyl acetate soluble fraction of the rhizomes of Caraganaconferta. Their structures have been assigned on the basis of spectroscopic studies [29]. |
| 5. Uvariahamiltonii: |
| Aurone is one of the several constituents isolated from the extracts of Uvariahamiltonii. The constituents were isolated by the way of a bioassay-guided fractionation based on DNA strand-scission and 9KB assays. While Aurone 1 appeared to be inactive in the 9KB assay it offered the greatest potency, of the compounds isolated, in the DNA strand-scission assay. In comparison to a bleomycin standard, compounds of this nature actually offer weak DNA strand-scission activity [30]. |
| 6.Pestalotiopsisphotiniae: |
| Photinides (1-6), six new unique benzofuranone-derived γ-lactones, have been isolated from the crude extract of the plant endophytic fungus Pestalotiopsisphotiniae. The structures of these compounds were elucidated primarily by NMR spectroscopy, and their absolute configurations were assigned by application of the CD excitation chirality method.Compounds1-6 displayed modest cytotoxic effects against the human tumor cell line MDA-MB-231 [31]. |
| 7. Rutagraveolens: |
| The voltage-gated potassium channel Kv1.3 constitutes an attractive pharmacological target for the treatment of effector memory T cell-mediated autoimmune diseases such as multiplesclerosis and psoriasis. Using 5-methoxypsoralen (1), a compound isolated from Rutagraveolens, as a template we previously synthesized 5-(4-phenoxybutoxy)psoralen (PAP-1, 2) which inhibits Kv1.3 with an IC50 of 2 nM. Since PAP-1 is more than 1000-fold more potent than 5-MOP, we here investigated whether attaching a 4-phenoxybutoxy side chain to other heterocyclic systems would also produce potent Kv1.3 blockers. While 4-phenoxybutoxy-substituted quinolines, quinazolines and phenanthrenes were inactive, 4- phenoxybutoxy-substituted quinolinones, furoquinolines, coumarins or furochromones inhibited Kv1.3 with IC50s of 150 nM to 10 mM in whole-cell patch-clamp experiments. Our most potent new compound is 4-(4-phenoxybutoxy)-7H-furo [3,2-g] chromene-7-thione (73, IC50 17 nM), in which the carbonyl oxygen of PAP-1 is replaced by sulfur. Taken together, our results demonstrate that the psoralen system is a crucial part of the pharmacophore of phenoxyalkoxypsoralen-type Kv1.3 blockers [32]. |
| 8. Artemisiacapillaris: |
| The title compound, 5,7-dihydroxy-2-(4-hydroxyphenoxy)-6-methoxy- chromen-4-one, is one of the constituents of (Japanese name, “Inchinko”), a Chinese folk medicine called as Capillarisin , is considered to play an important role for curing liver disorders and complications of diabetes [33]. |
| 9. Bidensparviflora: |
| Using the effects on histamine release of BidensparvifloraWilld. to identify biologically active compounds. Five chalcones, two flavanones and two aurones were isolated and identified as 2′-hydroxy-3, 4, 4′–trimethoxychalconee (I), 4, 2′,4′,6′- tetramethoxy dihydrochalcone (II), 3, 4, 2′, 4′, 6′-pentamethoxy dihydrochalcone (III), 7, 3′, 4′-trihydroxy flavanones (IV), 3′,4′dihydroxyflavanones 7-O-β-D-glucoside (V), 3, 4, 2′, -trihydroxychalcone 4′-O-β-D-glucoside (VI), 3-methoxy-4, 2′, 3′-trihydroxy chalcone 4′-O-β-D-glucoside (VII), sulfuretin 6-O-β-D-glucoside or 3′, 4′- dihydroxyaurone 6-O-β-Dglucoside( VIII), and maritimetin 7-O-β-D-glucoside or (6, 3′, 4′- trihydroxyaurones 7-O-β-D-glucoside(IX). Compounds IVII was obtained from BidensparvifloraWilld. For the first time, while compound III was a new dihydrochalcone, and I and II were isolated from natural sources for the first time. The chalconesglucosides and auronesglucosides exhibited activity in anti-allergic assays of histamine in ratmast cells induced by an antigen-antibody reaction and the inhibitory activity of NO production by macrophages is compared and discussed [34]. |
| 10. Sophoraflavescens: |
| Two novel lavandulyl flavonoids, (2S)-7-methoxyl-4’’, 5’’-dihydroxynorkurarinone (1) and (2S)-6’’- hydroxynorkurarinone-7-O-β-D-galactoside (2), were isolated from the rhizome of Sophoraflavescens. Their structures were elucidated by spectral methods, including 2D NMR spectroscopy. Both compounds showed cytotoxic activity against Hela cells, with 2 being moreactive than 1 [35]. |
| 11. Imperata cylindrical: |
| Bioactivity-guided fractionation of the methanolic extract of the rhizomes of Imperatacylindricaafforded a new compound, 5-hydroxy-2-(2-phenylethyl)chromone (1), together with three known compounds, 5-hydroxy-2-[2-(2- hydroxyphenyl)ethyl]chromone (2), flidersiachromone (3), and 5-hydroxy-2-styrylchromone (4). Among these four compounds, 1and 2 showed significant neuroprotective activity against glutamate-induced neurotoxicity in primary cultures of rat cortical cells [36]. |
| 12. Exophialapisciphila: |
| A new naturally occurring chromone derivative (7-methoxy-2,3,6-trimethylchromone)., along with two known indole alkaloids 3–4 were characterized from the ethylacetate extract of a soil-derived fungal strain, ExophialapisciphilaPHF-9. The structures of compounds 1–4 were established by detailed spectroscopic analysis and comparison with literature data. Compound was tested for its cytotoxicity against A-549, Hela, PANC-28 and BEL-7402 cell lines [37]. |
| 13. Humuluslupulus: |
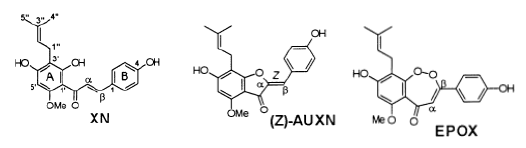
| Prenylated 2¢-hydroxychalcones and flavanones from the inflorescences of the female hopplant (Humuluslupulus) were shown to inhibit peroxynitrite-mediated oxidation of low-densitylipoproteins (LDL) at low micromolar concentrations. LDL oxidation was induced by theperoxynitrite generator, 3-morpholinosydnonimine (SIN-1), and measured by the formation ofconjugated dienes and thiobarbituric reactive substances. Human intake of prenylatedchalconesand flavanones is mainly through beer, which contains up to 4 mg/L of these polyphenols. Thetwo main oxidation products obtained by SIN-1 and peroxynitrite treatment of xanthohumol(XN), the principal prenylflavonoid of hops, were the aurone, auroxanthohumol (AUXN), andan endoperoxy derivative of XN, named endoperoxyxanthohumol (EPOX). In addition, thereaction produced smaller amounts of the nitro and nitroso derivatives of XN and EPOX. Theformation of these nitrated products was enhanced in the presence of sodium bicarbonate (25mM). SIN-1-induced formation of AUXN is considered to be a superoxide-mediated reaction,while the structure of EPOX points to a two electron oxidation reaction involving a Michaeltype addition with peroxynitrite as the nucleophile, followed by cyclization yielding a (1,2)-dioxepin-5-one ring structure. The flavanone isomer of XN, isoxanthohumol (IsoXN), unexpectedly showed a slight prooxidant effect instead of an inhibitory effect on LDL oxidation. Except for the formation of minor nitrated products, IsoXN remained largely unmodified upontreatment with SIN-1/peroxynitrite. Taken together, our results suggest that the R,â- unsaturated keto functionality of chalcones is most reactive toward superoxide and peroxynitrite anions [38]. |
| 14. Cyperus radians: |
| 3’, 4, 4’, 6-Tetramethoxyaurone is one of the several constituents that has been isolated from the methanolic extract of cyperus radians (cyperaceae). This cyperaceae produces large amounts of polymethylatedaurones and chromones as a part of a chemical defence system. Because both coumaran (dihydrobenzofuran) and chromene (benzopyran) are known to exhibit insect antifeedant activity, a similar corresponding relationship between the chemical structures of flavone (benzopyranone) and aurone (benzofuranone) suggests that aurones may show insect antifeedant activity. The naturally occurring aurone (3’, 4, 4’, 6-tetramethoxyaurone) showed insect antifeedant activity against Spodopteralitura larvae and was found to be most active at an ED50 of 0.12μmol/cm2 [39]. |
 |
| 15. Bidensfrondosa: |
| Maritimetin (3’,4’,6,7-tetrahydroxyaurone), a compound that has been isolated from Bidensfrondosa was screened for their antioxidant potential toward 1,1-diphenyl-2-picrylhydrazyl (DPPH) and superoxide anion radicals [40]. |
| 16.Dipteryxodorata: |
| A new cassanediterpene, dipteryxic acid (1), and a new isoflavonolignan, 5-methoxyxanthocercin A (2), as well as four known active compounds, isoliquiritigenin (3), 6,4¢-dihydroxy-3¢-methoxyaurone (4), sulfuretin (5), and (()-balanophonin (6), and five known inactive compounds, butin, eriodictyol, 7-hydroxychromone, 7,3¢-dihydroxy-8,4¢- dimethoxyisoflavone, and (-)-lariciresinol, were isolated from an ethyl acetate-soluble extract of the seeds of Dipteryxodorata, using a bioassay based on the induction of quinonereductase (QR) in cultured Hepa 1c1c7 mouse hepatoma cells to monitor chromatographic fractionation. The structures of compounds 1 and 2 were elucidated by spectroscopic data interpretation.Single-crystal X-ray diffraction analysis was used to confirm the relative stereochemistry of compound 1. Selected compounds (3-5) were evaluated in a mouse mammary organ cultureassay, with isoliquiritigenin (3) found to exhibit 76% inhibition at a dose of 10 íg/mL [41 |
| 17. Cotinuscoggygria: |
| Six constituents (1-6) were isolated from EtOAc-soluble partitions of two separate collections of the whole plants of Cotinuscoggygria, namely, disulfuretin {2,2¢-[1,2-bis(3,4-dihydroxyphenyl)-1,2-ethanedylidene]-bis[6-hydroxy-3(2H)- benzofuranone] (1)}, sulfuretin (2), sulfurein (3), gallic acid (4), methyl gallate (5), and pentagalloyl glucose (6). The structure of the novel biaurone1 was determined by spectral and chemical methods. Compounds 1-6 were found to be potent antioxidants in a 1, 1-diphenyl-2-picrylhydrazyl free-radical scavenging assay [42]. |
IV. CONCLUSION |
| Nutrition security goes beyond food security by considering a community’s access to essential nutrients, not just calories. Production of pharmaceuticals in plants for therapeutic purposes shows great promise, with some clinical trials and many others under investigation. Plant production systems are easily expanded and typically provide a lower cost of production relative to the cell culture systems currently used to produce biological therapeutics. Different agencies across the globe are actively developing the agronomic and manufacturing regulations needed to ensure safety, consistency, and potency of plant-made pharmaceuticals. |
References |
|