ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Majdouline Larif1, Ali Zahlou2, Samir Chtita3, Lahcen Bejjit4, Mohammed Bouachrine4, Tahar Lakhlifi5*
|
| Corresponding Author: Tahar Lakhlifi, |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Fatty acid synthase (FAS), an essential enzyme for de novo lipogenesis, has been implicated in a number of disease states, including obesity, dyslipidemia and cancer. The research in new pi-conjugated molecules with specific applications has become one of the most interesting topics in the fields of chemical physics and materials science. The use of low band gap materials is a viable method for a better harvesting of the solar spectrum and an improved raise of its efficiency. The control of this parameter of these materials is a research issue of ongoing interest. In this work a quantum chemical investigation has been performed to explore the optical and electronic properties of a series of different compounds based on 3-aryl-4-hydroxyquinolin-2-(1H)-one. Different electron side groups were introduced to investigate their effects on the electronic structure. The theoretical knowledge of the HOMO and LUMO energy levels of the components is basic in studying organic solar cells so the HOMO, LUMO and energy Gap of the studied compounds have been calculated and reported. These properties suggest these materials as good candidates for organic solar cells.
Keywords |
| Conjugated molecules, Organic solar cells, DFT, Low band-gap, Electronic properties. |
INTRODUCTION |
| FAS are down regulated in most normal human tissues because of the fat in our diet, with the exception of lactating breast and cycling endometrium. In contrast, FAS is often highly expressed in human cancers (breast, prostate, colon, ovary, endometrium and thyroid). This differential tissue distribution makes FAS an attractive target for cancer cells [1,2]. Therefore designing and synthesizing molecules with interesting properties play a crucial role in technology. At the same time it is important to understand the nature of the relationship between the molecular structure and the electronic properties to provide guidelines for the development of new materials. Recent work in this area has been focused on the synthesis and design of new molecules combining several conjugated systems with narrow band gap [3]. A fundamental understanding of the ultimate relations between structure and properties of these materials is necessary to benefit from their adaptative properties to photovoltaic cells. In parallel with this experimental work on these new materials, theoretical efforts have indeed begun to constitute an important source of valuable information which complements the experimental studies, thereby contributing to the understanding of the molecular electronic structure as well as the nature of absorption and photoluminescence [4]. Hetero-junctions between organic semiconductors are central to the operation of light-emitting and photovoltaic diodes. [5]. A common strategy to enhance the power conversion of the photovoltaic device efficiency, by improving the exciton charge transfer and transport, is the development of low band gap conjugated materials as an alternating donor (D)-acceptor (A) structure in the main polymer backbone [6]. The 3-aryl-4-hydroxyquinolin-2-(1H)-one and its derivatives still receive considerable attention for their exceptional optoelectronic properties especially photoconductive ones [7]. In the course of a program on the synthesis of new organic materials with useful electronic properties and in order to elucidate further the effect of nitrogen atom ring substitution as well as variation of ring substituents on the relative photoconductivity, a number of various 3-aryl-4-hydroxyquinolin-2-(1H)-one (Figure 1) were prepared by [8]. These materials offer many advantages in terms of easy synthesis and purification, and generally exhibit high relative photoconductivity. In this paper, eleven compounds based on 3-aryl-4-hydroxyquinolin-2-(1H)-one (M1, M2, M3, M4, M5, M6, M7, M8, M9, M10, M11, M12, M13, M14, and M15, shown in figure 1), are designed. The geometries, electronic properties, absorption and emission spectra of these studied compounds are studied by using density functional theory (DFT) and timedependent density functional theory (TD/DFT) with the goal to find potential sensitizers for use in organic solar cells. |
TRAINING SET |
| All compounds studied in this work are 3-aryl-4-hydroxyquinolin-2-(1H)-one derivatives. This data set, containing Fifteen compounds (Table 1 and Figure 1), one of the biggest sets of human FAS type I inhibitors available in literature, was described and the biological activity was investigated by Rivkin and co-workers [8].Therefore, it was selected for this study. The biological activity was measured according to the concentration (in nanomolar, nM) required for fifty percent inhibition of the Human FAS enzymatic activity, IC50. The observed IC50 measurements were converted into their corresponding log IC50 (or pIC50) and are also presented in table 1. Fifteen compounds present the exact values of their respective IC50. |
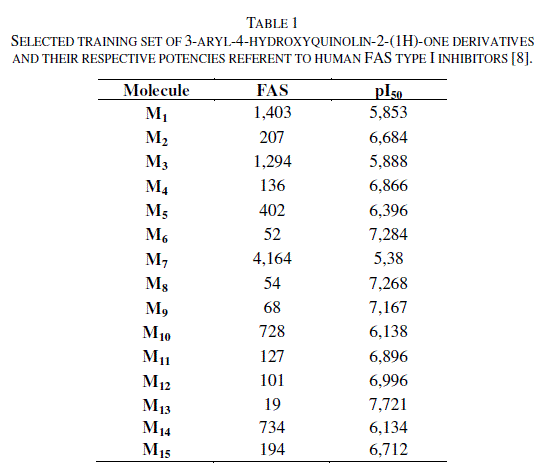 |
 |
| Theoretical methodology |
| DFT method of three-parameter compound of Becke (B3LYP) [9] was used in all the study of the neutral and polaronic compounds. The 6-31G (d) basis set was used for all calculations [10]. To obtain the charged structures, we start from the optimized structures of the neutral form. The calculations were carried out using the GAUSSIAN 03 program [11]. The geometry structures of neutral and doped molecules were optimized under no constraint. We have also examined HOMO and LUMO levels; the energy Egap is evaluated as the difference between the HOMO and LUMO energies. The ground state energies and oscillator strengths were investigated using the ZINDO/s, calculations on the fully optimized geometries. In fact, these calculation methods have been successfully applied to other conjugated polymers [12]. |
RESULTS AND DISCUSSION |
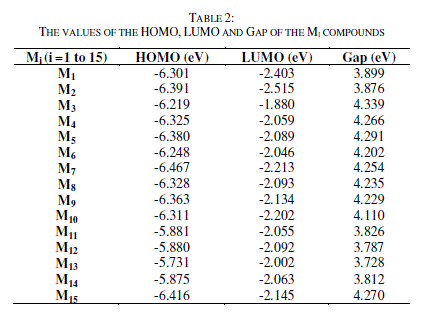
| The results of the optimized structures obtained by B3LYP/6-31G(d) (Figure 2) show that they have similar conformations(quasi planar conformation).We found that the modification of several groups does not change the geometric parameters. Table 2 lists the calculated frontier orbital energies and energy Gap between highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) and the Gap energy of the studied molecules. |
 |
 |
 |
 |
| Electronic parameters |
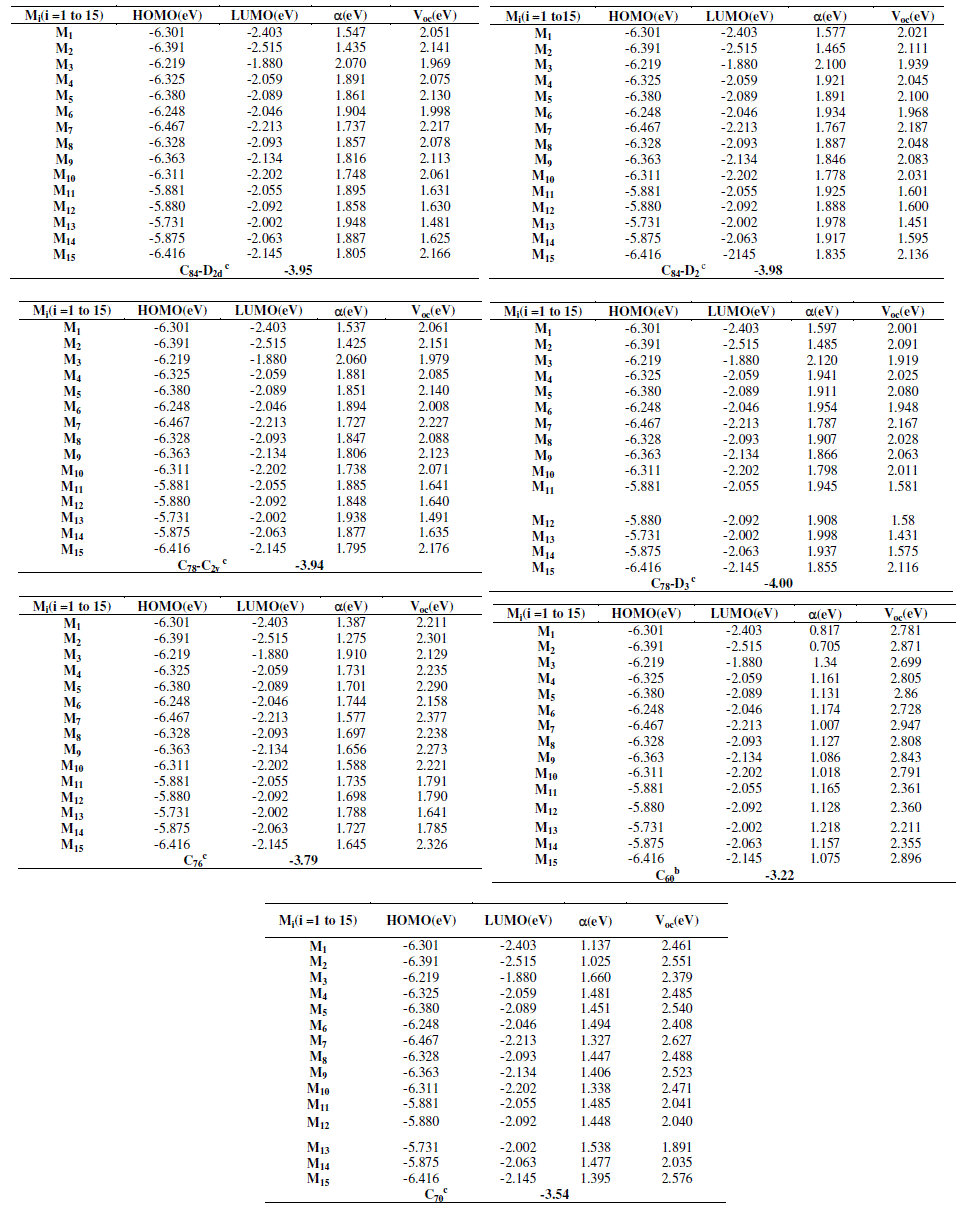
| Electronic structures are fundamental to the interpretation and understanding of the absorption spectra. It is deduced that the substitution, of 3-aryl-4-hydroxyquinolin-2-(1H)-one by several chemical groups, push up/down the HOMO/LUMO energies in agreement with their electron acceptor/donor character. As shown in table 2, the HOMO and LUMO energies of 1 to 13 change significantly, the LUMOs for molecules M1, M2, M3, M4, M5, M6, M7, M8, M9, M10, M11, M12, M13, M14 and M15 are located at (-2.403; -2.515; -1.880; -2.059; -2.089; -2.046; -2.213: -2.093; -2.134; - 2.202; -2.055; -2.092; -2.002; -2.063 and -2.145) eV, respectively. The HOMOs for M1, M2, M3, M4, M5, M6, M7, M8, M9, M10, M11, M12, M13, M14 and M15 are located at (-6.301; -6.391; -6.219; -6.325; -6.380; -6.248; -6.467; -6.328; - 6.363; -6.311; -5.881; -5.880; -5.731; -5.875 and -6.416) eV respectively. It can also be found that, the HOMO and LUMO energies of the studied compounds are slightly different. This implies that different structures play key roles on electronic properties and the effect of slight structural variations, especially the effect of the motifs branched to the ring on the HOMO and LUMO energies is clearly seen. In addition, the energies of Gap of the studied molecules differ slightly from 3.728 eV to 4.339 eV depending on the different structures. They are classified in the following order M3 > M6 > M1 > M10 > M4 > M8> M9 > M5 > M2 > M15 > M7 > M13 > M14> M12> M11. The photovoltaic process can be summarized as follows: |
 |
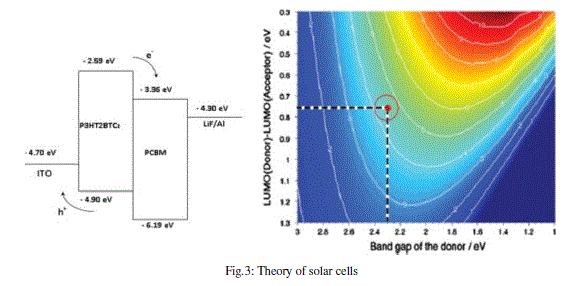
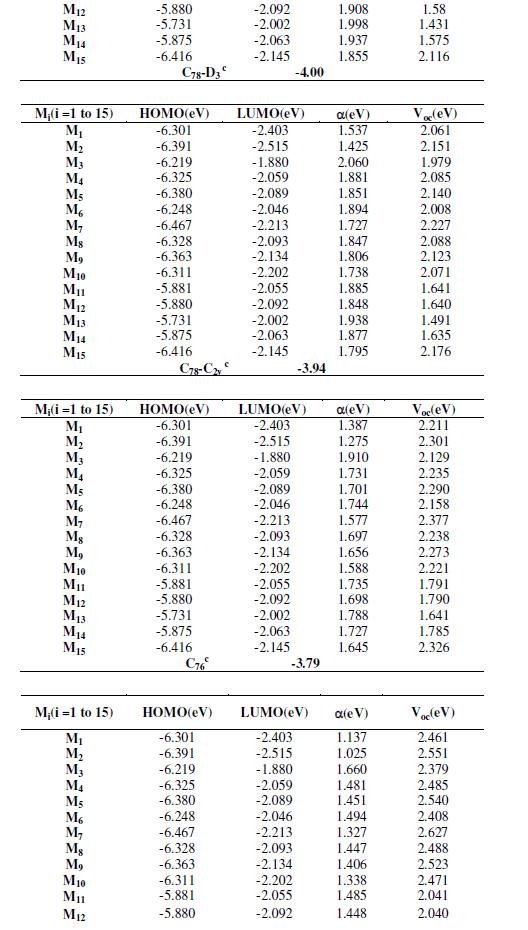
| On the other hand and from the above analysis, we know that LUMO energy levels of the twelve studied molecules are much higher than that of ITO conduction band edge (−4.7 eV). Thus, molecules in excited states of the studied molecules have a strong ability to inject electrons into ITO electrodes. The experiment phenomenon is quite consistent with previous literature [13], this latter reported that the increase of the HOMO levels may suggest a negative effect on organic solar cell performance due to the broader gap between the HOMO level of the organic molecules and the HOMO level of the acceptor derivatives from PCBM (C60, C70, C76, C78-C2V, C78-D3, C84-D2) (Figure 4). |
 |
| As shown in table 2, both HOMO and LUMO levels of the studied molecules agree well with the requirement for an efficient photosentizer. It should be noted that the LUMO levels of the studied compounds Mi (i=1-15) are higher than that of PCBM derivatives which varies in literature from -4.0 to -3.47 eV (C60 (-3.47 eV); C70 (-3.54), C76 (-3.79), C78- C2V (-3.94), C78-D3 (-4.0), C84-D2 (-3.98) [14]. To evaluate the possibilities of electron, transfer from the studied molecules to the conductive band of the proposed acceptors, the HOMO and LUMO levels are compared. Knowing that in organic solar cells, the open circuit voltage is found to be linearly dependent on the HOMO level of the donor and the LUMO level of the acceptor. The maximum open circuit voltage (Voc) of the BHJ solar cell is related to the difference between the highest occupied molecular orbital (HOMO) of the donor (our studied molecules) and the LUMO of the electron acceptor (PCBM derivatives in our case), taking into account the energy lost during the photocharge generation [15]. The theoretical values of open-circuit voltage Voc have been calculated from the following expression: |
 |
| The obtained values of Voc of the studied molecules calculated according to the equation (1) range from 1.407 eV to 2.603 eV (see tables 3), these values are sufficient for a possible efficient electron injection. Therefore, all the studied molecules can be used as sensitizers because the electron injection process from the excited molecule to the conduction band of PCBM derivatives and the subsequent regeneration is possible in an organic sensitized solar cell. |
 |
 |
 |
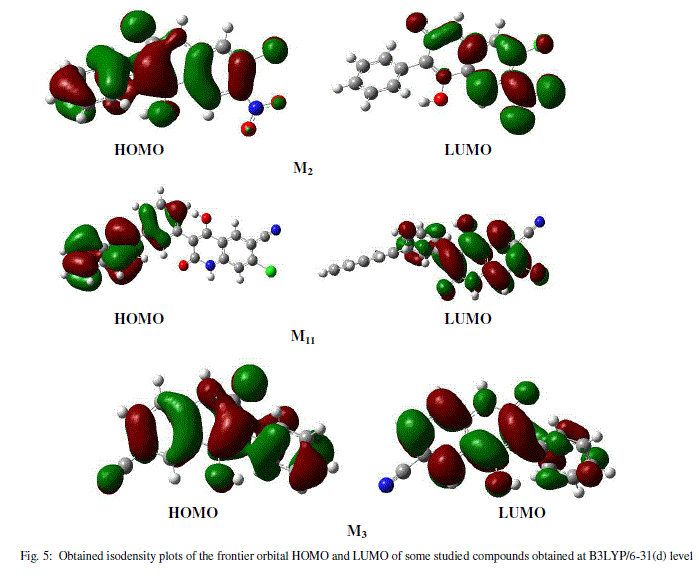
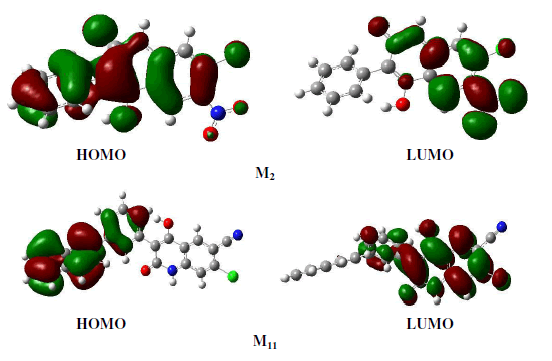
| The frontier molecular orbital (MO) contribution is very important in determining the charge-separated states of the studied molecules because the relative ordering of occupied and virtual orbital provides a reasonable qualitative indication of excitations properties [16]. In general, as shown in figure 5 (LUMO, HOMO), the HOMOs of these molecules in the neutral form possess a π-bonding character within subunit and a π-antibonding character between the consecutive subunits while the LUMOs possess a π-antibonding character within subunit and a π-bonding character between the subunits whereas it is the opposite in the case of doped forms. |
 |
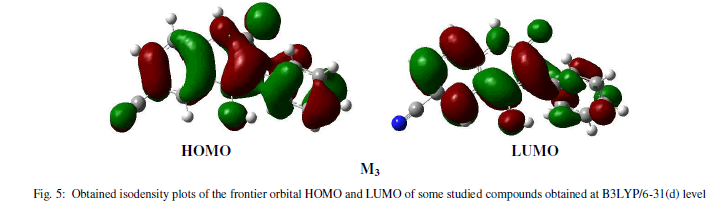 |
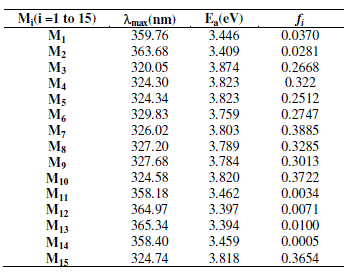
| On the other hand and how the absorption of a new material matches with the solar spectrum is an important factor for the application as a photovoltaic material, and a good photovoltaic material should have broad and strong visible absorption characteristics. In fact, we have calculated the UV-Visible spectra of the studied compounds using TD-DFT starting with optimized geometry obtained at B3LYP/6-31(d) level. As illustrated in table 4, we can find the values of calculated wavelength ïÃÂìmax and oscillator strengths O.S. Excitation to the S1 state corresponds almost exclusively to the promotion of an electron from the HOMO to the LUMO orbital. The absorption wavelengths arising from S0→S1 electronic transition increase progressively with the increasing of conjugation lengths. It is reasonable, since HOMO→LUMO transition is predominant in S0→S1 electronic transition; the results are a decrease of the LUMO and an increase of the HOMO energy. Data in table 4 shows that there is a bathochromic shift when passing from Molecule M3 (320.05 nm) to Molecule M13 (365.34 nm). This effect is obviously due to the aromaticity, conjugaison and substitution effects in the several studied compounds. Those interesting points are seen both in the studying the electronic and absorption properties. |
 |
CONCLUSION |
| This study is a theoretical analysis of the geometries and electronic properties of various compounds based on 3-aryl-4- hydroxyquinolin-2-(1H)-one which displays the effect of substituted groups on the structural and opto-electronic properties of these materials and leads to the possibility to suggest these materials for organic solar cells application. The concluding remarks are: * The results of the optimized structures for all studied compounds so that they have similar conformations (quasi planar conformation). We found that the modification of several groups does not change the geometric parameters. |
| The calculated frontier orbital energies HOMO and LUMO and energy Gap showed that the energy Gap of the studied molecules differ slightly from 3.728 eV to 4.339 eV depending on the different structures. |
| * The energy Gap of M11 is much smaller than that of the other compounds. |
| * All the studied molecules can be used as sensitizers because the electron injection process from the excited molecule to the conduction band of PCBM and the subsequent regeneration are feasible in the organic sensitized solar cell. |
| * This calculation procedure can be used as a model system for understanding the relationships between electronic properties and molecular structure and also can be employed to explore their suitability in electroluminescent devices and in related applications. Presumably, the procedures of theoretical calculations can be employed to predict and assume the electronic properties on yet prepared and efficiency proved the other materials, and further to design new materials for organic solar cells. |
ACKNOWLEDGEMENTS |
| We are grateful to the “Association Marocaine des Chimistes Théoriciens” (AMCT) for its pertinent help concerning the programs |
References |
|