ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
A.S. Fouda 1, K. Shalabi1, A.E.El-Shennawi2, R.M. Abo Shohba2, A.A.El-Naggar2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The corrosion inhibition of carbon-steel in 1 M HCl by some (Z)-N'-(1-phenylethylidene) benzohydrazide derivatives (PEBH) has been investigated using weight loss method, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. Results obtained showed that these investigated compounds are good inhibitors and their inhibition efficiencies increase with increasing inhibitor concentration and decrease with increase in temperature. Moreover, polarization studies clearly revealed that the presence of inhibitors changes the mechanism of hydrogen evolution and the metal dissolution i.e. they act as mixed type inhibitors. EIS study showed that charge transfer resistance increases with the increase of inhibitor concentration but the capacitance of double layer decreases. The adsorption of investigated compounds obeys Langmuir’s adsorption isotherm. The activation parameters were determined and discussed. The mechanism of adsorption had been explained on the basis of chemical structure of the investigated inhibitors. It was found that there is a good agreement between the different tested techniques.
Keywords |
| Carbon steel, HCl, potentiodynamic polarization, EIS, EFM. |
INTRODUCTION |
| Carbon steel is an alloy of iron it is widely used in petrochemical, chemical and metallurgical industries. It is also used as a construction material owing to its excellent mechanical properties and cost effectiveness. However, it is easily undergoes corrosion in various environmental conditions. Acid solutions are widely used in industry, the most important fields of application being acid pickling, industrial acid cleaning, acid descaling and oil well acidising, because of the general aggressiveness of acid solutions, the practice of inhibition is commonly used to reduce the corrosive attack on metallic materials. Inhibitors are generally used for this purpose to control the metal dissolution. Most of the well-known inhibitors are organic compounds containing nitrogen, sulphur and /or oxygen atoms. It has been observed that the most of the organic inhibitors act by adsorption on the metal surfaces [1]. The adsorption of corrosion inhibitor depends mainly on physico-chemical properties of the molecules such as functional group, steric factor, molecular size, molecular weight, molecular structure, aromaticity, electron density at the donor atoms and π- orbital character of donating electrons [2-6], and also, on the electronic structure of molecules [7-10] The aim of the present work is to study the inhibitive action of some new (Z)-N'-(1-phenylethylidene) benzohydrazide derivatives (PEBH) towards the corrosion of C-steel in 1 M hydrochloric acid solution using chemical and electrochemical techniques. Moreover, the effect of temperature on the dissolution of carbon steel, as well as, on the inhibition efficiency of the studied compounds was also investigated and discussed. |
II. EXPERIMENTAL |
| 2.1. Materials and reagents Carbon steel strips (BDH grade) containing (weight %): 0.2 C, 0.024 P, 0.003 Si, 0.35 Mn, and rest Fe were used in this investigation. All chemicals used were of AR grade. Specimens of C-steel strips were abraded successively by emery papers of different grades, i.e. 320, 400 ,600, 800 and 1000 finally polished with a 4/0 emery paper to obtain mirror like finish, then degreased ultrasonically with ethyl alcohol and rinsed with bidistilled water several times and dried between two fitter papers. AR grade hydrochloric acid (37 %) was used for preparing the corrosive solutions. |
| Appropriate concentration of aggressive solutions used (1 M HCl) was prepared using bidistilled water. The structures of the investigated compounds are shown below [11]: |
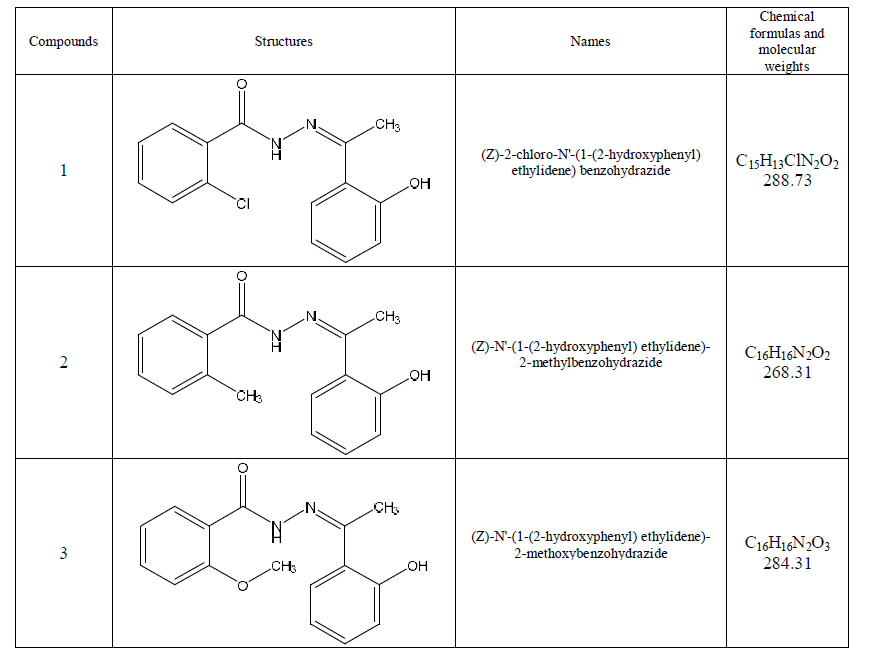 |
| 2.2. Weight loss measurements For weight loss measurements, rectangular C-steel specimens of size 20 x 20 x 2 mm were immersed in 100 ml inhibited and uninhibited solutions and allow to stand for several intervals at 25°C in water thermostat. Therefore, the weight losses given by: Δm = (m1-m2) (1) where m1 and m2 are the weights of metal before and after exposure to the corrosive solution, respectively. The percentage inhibition efficiency (% IE) and the degree of surface coverage (θ) of the investigated compounds were calculated using equation (2): % IE = θ x 100 = [1 – ( Δminh/ Δmfree)] x 100 (2) where Δmfree and Δminh are the weight losses per unit area in the absence and presence of additives, respectively. 2.3 Electrochemical measurements A three electrode electrochemical cell was used. The working electrode was C-steel of surface area of 1 cm2. Before each experiment, the electrode was abraded using emery papers as before. After this, the electrode was cleaned ultrasonically with ethyl alcohol and washed by bidistilled water. All potentials were given with reference to the saturated calomel electrode (SCE). The counter electrode was a platinum plate of surface area of 1 cm2. The working electrode was immersed in the test solution during 30 min until a steady state open circuit potential (Eocp) was obtained. The polarization curves were recorded by polarization from -0.6 V to 0.2 V under potentiodynamic conditions corresponding to 1 mV/s (sweep rate) and under air atmosphere. All measurements were carried out with C-steel electrode in 1 M HCl in the absence and presence of different concentrations (15 x 10-6 – 27 x 10-6 M) of the investigated inhibitors at 25 °C. All experiments were carried out at 25 ± 1 °C. The inhibition efficiency and surface coverage (θ) were calculated from equation (3): |
| % IE = θ x 100 = [1 – (icorr(inh.)/ icorr(free))] x 100 (3) |
| where icorr(free) and icorr(inh) are the corrosion current densities in the absence and presence of inhibitor, respectively. Electrochemical impedance spectroscopy measurements were performed using the same cell that used in polarization experiments. The EIS carried out over a frequency range of 1 Hz to 100 kHz, with a signal amplitude perturbation of 10 mV. The inhibition efficiency (% IE) and surface coverage (θ) of the investigated compounds obtained was calculated from equation (4): |
| % IE = θ x 100 = [(Rct - R°ct) / Rct] x 100 (4) |
| where Rºct and Rct are the charge transfer resistance values in the absence and presence of the inhibitors, respectively. Electrochemical frequency modulation is a non-destructive technique as electrochemical impedance spectroscopy that can directly and rapidly give values of the corrosion current without a prior knowledge of Tafel constants. The great of the EFM is the causality factors, which serves as an internal check on the validity of the EFM measurement. With the causality factors the experimental EFM data can be verified [12-13]. Identical cell assembly was used as in impedance studies. All electrochemical measurements were carried out using Potentiostat /Galvanostat / Zra analyzer (Gamry PCI300/4). A personal computer with DC105 software for potentiodynamic, EIS300 software for EIS and EFM140 software for EMF and Echem Analyst 5.21 was used for data fitting. |
3. RESULTS AND DISCUSSION |
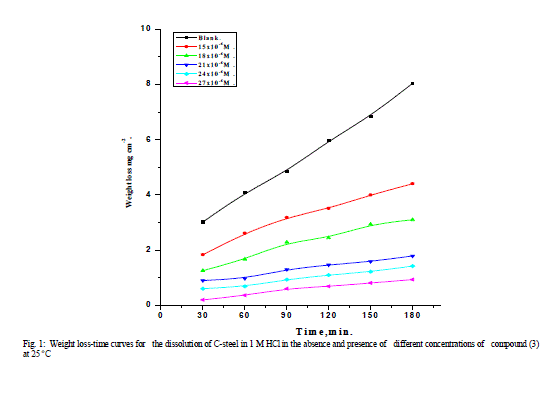
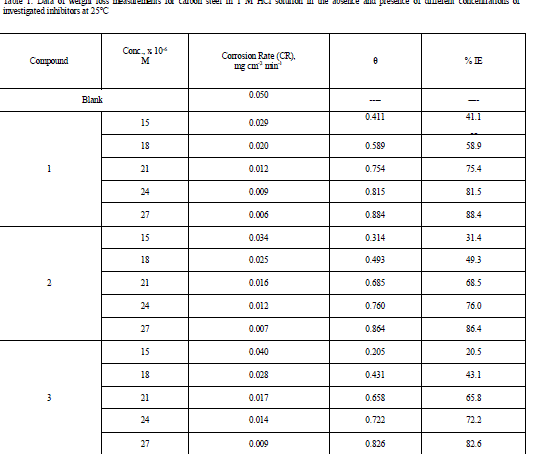
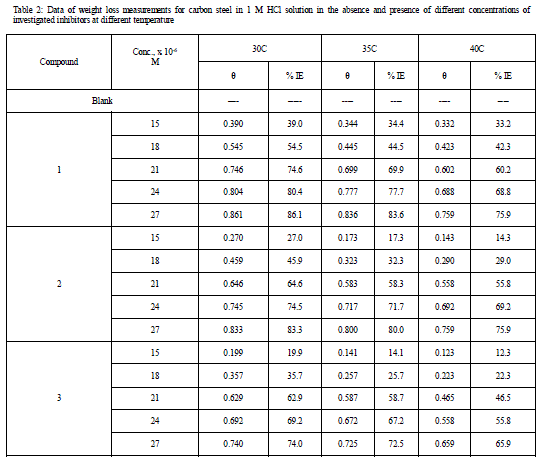
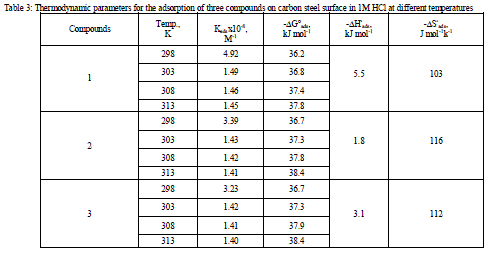
| 3.1 Weight – loss measurements Figure 1 shows the effect of concentration of compound (3) on the weight loss vs. time of C-steel at 25°C. Similar curves were obtained for the other two compounds not shown. It is obvious that the weight loss of C-steel in presence of inhibitors varies linearly with time, and is much lower than that obtained in blank solution. The linearity obtained indicated the absence of insoluble surface film during corrosion and that the inhibitors were first adsorbed onto the metal surface and, therefore, impede the corrosion process [14]. The calculated values of the percentage inhibition efficiency (% IE) at different concentrations of investigated compounds in 1 M HCl at different temperatures (25- 40°C) are given in Tables 1and 2. From these Tables, the inhibition efficiencies increase by increasing inhibitors concentrations and decreases by increasing in temperature. This behavior could be attributed to the increase of the number of adsorbed molecules at the metal surface. At one and the same inhibitor concentration % IE increases in the following order: (1) < (2) < (3). |
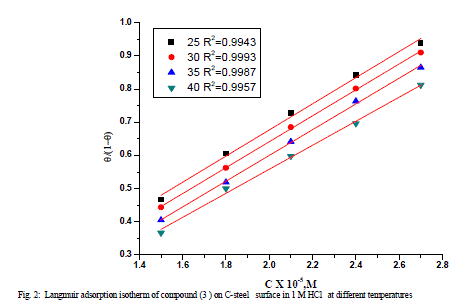
| 3.2 Adsorption isotherm Basic information on the interaction between the inhibitors and the C-steel can be provided by the adsorption isotherm. Two main types of interaction can describe the adsorption of the organic compound: physical adsorption and chemical adsorption. These are influenced by the chemical structure of the inhibitor, the type of the electrolyte, the charge and nature of the metal. The surface coverage, θ, of the metal surface by the adsorbed inhibitor was evaluated from weight loss measurements using equation (2). The θ values of different inhibitor concentrations at 25 °C were tested by fitting to various isotherms including, Frumkin, Langmuir, Temkin and Flory-Huggins. By far the best fit was obtained with the Langmuir isotherm is given as [15]: |
| θ /1-θ = Kads C (5) |
| where C is the inhibitor concentration and Kads is the equilibrium constant of adsorption process and is related to the standard free energy of adsorption ΔGÃâ¹ÃÅ¡ads by Eq. (6) : Kads = 1/55.5 exp (-ΔG° ads/RT) (6) The value of 55.5 is the concentration of water in solution expressed in mole per liter, R is the universal gas constant and T is the absolute temperature . |
| A plot of (θ /1-θ) against C, for all concentrations of inhibitors (Figure 2) a straight line relationship was obtained in all cases with correlation coefficients (R2) in more than 0.994. The deviation of the slope from unity as observed from this study could be interpreted that there are interactions between adsorbed species on the metal surface as well as changes in adsorption heat with increasing surface coverage [16, 17], factors that were ignored in the derivation of Langmuir isotherm. The negative ΔG°ads values (Table 3) are consistent with the spontaneity of the adsorption process and the stability of the adsorbed layer on the C-steel surface [18]. It is generally accepted that the values of ΔG°ads up to -20 kJ mol-1 the types of adsorption were regarded as physisorption , the inhibition acts due to the electrostatic interaction between the charged molecules and the charged metal, while the values around -40 kJ mol-1 or smaller, were seen as chemisorptions, which is due to the charge sharing or a transfer from the inhibitor molecules to the metal surface to form covalent bond [19-20]. The ΔG°ads values obtained in this study range from – 36.2 to – 36.7 kJ mol-1. It suggested that the adsorption mechanism of investigated inhibitors on C-steel in 1 M HCl solution was typical of physisorption. |
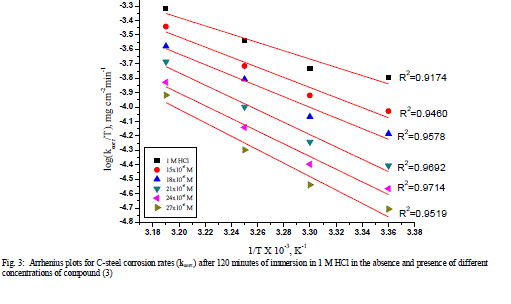
| 3.3 Kinetic-thermodynamic corrosion parameters As noticed previously, the adsorption process was well elucidating by using a thermodynamic model, in addition a kinetic-thermodynamic model was another tool to explain the mechanism of corrosion inhibition for an inhibitor. The apparent effective activation energies (E* a) for the corrosion reaction of C-steel in HCl in the absence and presence of different concentrations of investigated compounds were calculated from Arrhenius-type equation [21]: |
| k=A exp (-Ea * / RT) (7) |
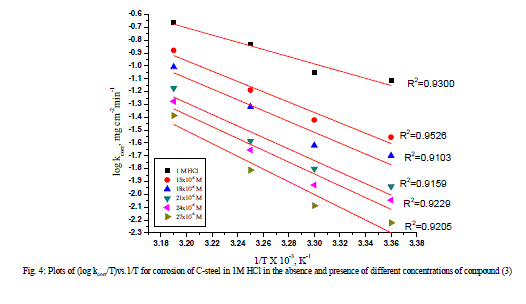
| where A is the Arrhenius pre-exponential factor. A plot of log k (corrosion rate) vs. 1 / T gave straight lines as shown in (Figure 3). The entropy of activation (ΔS*) and the enthalpy of activation (ΔH*) for the intermediate complex in the transition state for the corrosion of C-steel in HCl in the absence and presence of different concentrations of investigated compounds were obtained by applying the transition-state equation [22-24] |
| k = RT / Nh exp (ΔS*/R) exp (-ΔH* / RT) (8) |
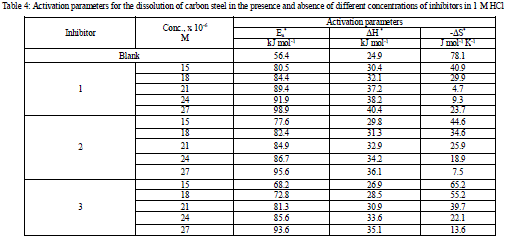
| where h is the Planck’s constant and N is the Avogadro’s number A plot of log k (corrosion rate) / T vs. 1 / T should give a straight lines (Figure 4), with a slope of (-ΔH* / 2.303R), and an intercept of [(log( RT / Nh ) + (ΔS*/2.303R)] [25-26], from which the values of ΔH* and ΔS* were calculated, respectively. (Table 4) exhibited values of apparent activation energy, apparent enthalpies ΔH* and entropies ΔS* for Csteel dissolution in 1 M HCl solution in the absence and presence of different investigated compounds. The presence of inhibitors decreased the activation energies of C-steel indicating strong adsorption of the inhibitor molecules on the metal surface and the presence of these additives induces the adsorption of theses additives on the surface of C-steel. Values of the entropy of activation ΔS* in the absence and in presence of the studied compounds are negative .This implies that the activated complex in the rate determining step represents an association rather than a dissociation step [27]. This means that the activated molecules were in higher order state than that at the initial stage [28-29]. |
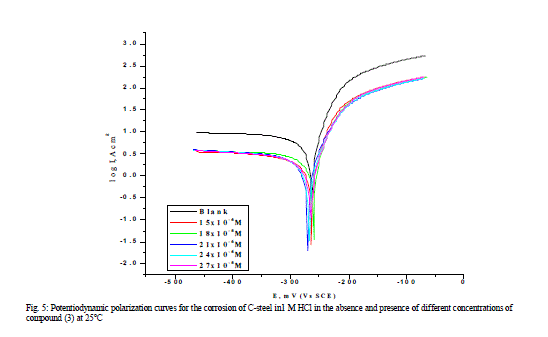
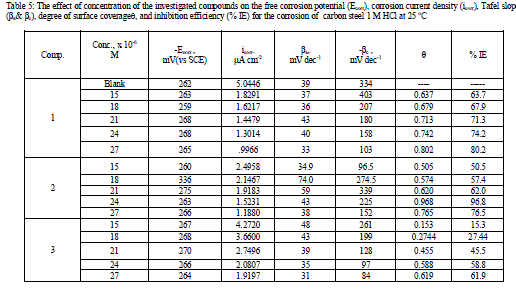
| 3.4 Polarization curves Figure.5 illustrates the polarization curves of carbon steel in 1 M HCl solution without and with various concentrations of compound (3) at 25Ãâ¹ÃÅ¡C. The presence of investigated compounds shift both anodic and cathodic branches to the lower values of corrosion current densities and thus causes a remarkable decrease in the corrosion rate. The parameters derived from the polarization curves in (Figure 5) are given in Table 5. In 1 M HCl solution, the presence of the investigated compounds cause a remarkable decrease in the corrosion rate i.e., shifts both anodic and cathodic curves to lower current densities. In other words, both cathodic and anodic reactions of carbon steel electrode are retarded by the investigated compounds in hydrochloric acid solution. The Tafel slopes of βa and βc at 25°C do not change remarkably upon addition of the investigated compounds, which indicates that the presence of investigated compounds does not change the mechanism of hydrogen evolution and the metal dissolution process. Generally, an inhibitor can be classified as cathodic or anodic type if the shift of corrosion potential in the presence of the inhibitor is more than 85 mV with respect to that in the absence of the inhibitor [30, 31]. In the presence of investigated compounds, Ecorr shifts to less negative but this shift is very small (about 20-30 mV), which indicates that investigated compounds can be arranged as a mixed-type inhibitor, with predominant anodic effectiveness. The % inhibition increased with increasing the concentration of the compounds, the inhibition efficiency of the three tested compounds measured by polarization method increased in the following order: (1) < (2) < (3). This sequence is in accordance with that obtained from weight-loss measurements. |
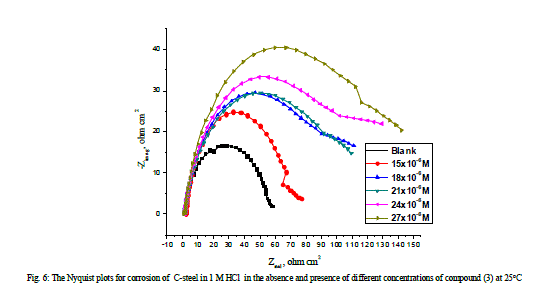
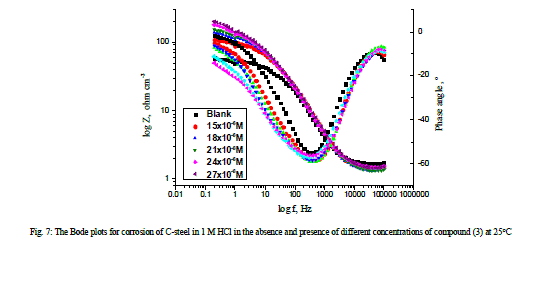
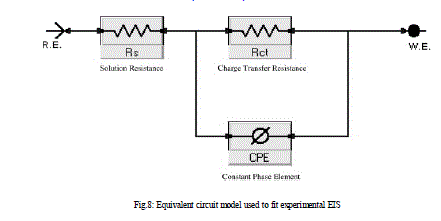
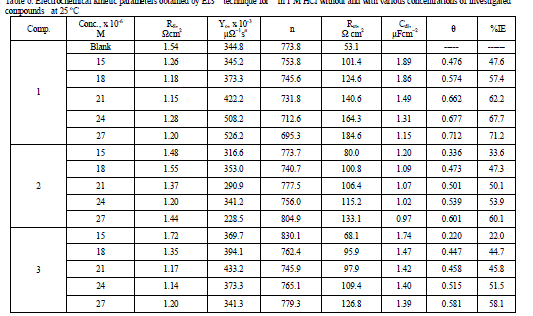
| 3.5 Electrochemical impedance spectroscopy Figures 6 and 7 show the Nyquist and Bode diagrams of carbon steel in 1 M HCl solutions containing different concentrations of compound (3) at 25°C. All the impedance spectra exhibit one single depressed semicircle. The diameter of semicircle increases with the increase of the investigated compounds concentration. The impedance spectra exhibit one single capacitive loop, which indicates that the corrosion of steel is mainly controlled by a charge transfer process [32] and the presence of the investigated compounds does not change the mechanism of carbon steel dissolution [33]. In addition, these Nyquist diagrams are not perfect semicircles in 1 M HCl that can be attributed to the frequency dispersion effect as a result of the roughness and inhomogeneous of electrode surface [34]. Furthermore, the diameter of the capacitive loop in the presence of inhibitor is larger than that in the absence of inhibitor (blank solution), and increased with the inhibitor concentration. This indicates that the impedance of inhibited substrate increased with the inhibitor concentration. [33,34]. This behavior is usually attributed to the inhomogeneity of the metal surface arising from surface roughness or interfacial phenomena [35], which is typical for solid metal electrodes [36]. Generally, when a non-ideal frequency response is presented, it is commonly accepted to employ the distributed circuit elements in the equivalent circuits. What is most widely used is the constant phase element (CPE), which has a noninteger power dependence on the frequency [37]. Thus, the equivalent circuit depicted in Figure 8 is employed to analyze the impedance spectra, where Rs represents the solution resistance, Rct denotes the charge-transfer resistance, and a CPE instead of a pure capacitor represents the interfacial capacitance. The impedance of a CPE is described by the equation 9: |
| ZCPE = Y0 -1 (jω)-n (9) |
| where Y0 is the magnitude of the CPE, j is an imaginary number, ω is the angular frequency at which the imaginary component of the impedance reaches its maximum values, and n is the deviation parameter of the CPE: -1 ≤ n ≤ 1. The values of the interfacial capacitance Cdl can be calculated from CPE parameter values Y0 and n using equation 10 [38]: Cdl = Y0 (ωmax) n-1 (10) The values of the parameters such as Rs, Rct, through EIS fitting as well as the derived parameters Cdl and η % are listed in Table 6. The order of inhibition efficiency obtained from EIS measurements is as follows: (1) < (2) < (3). This sequence is in accordance with that obtained from weight-loss and polarization measurements. |
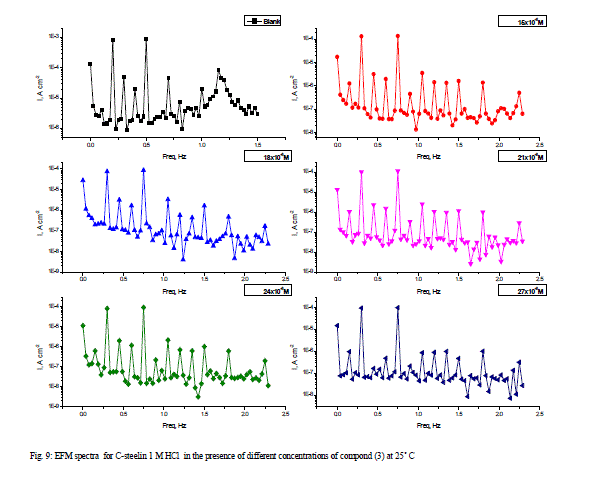
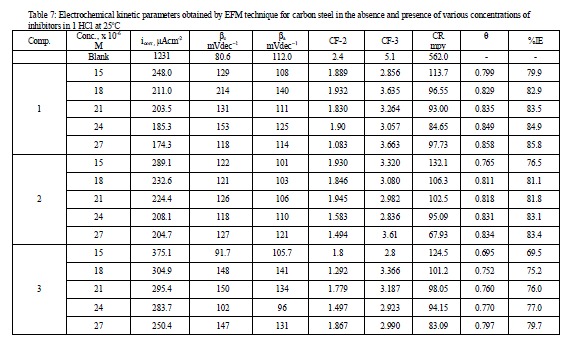
| 3.6 Electrochemical Frequency Modulation Technique (EFM) The EFM is a nondestructive corrosion measurement technique that can directly give values of the corrosion current without prior knowledge of Tafel constants. Like EIS, it is a small AC signal. Intermodulation spectra obtained from EFM measurements of carbon steel in 1 M HCl solution, in the absence and presence of different concentrations of compound (3) at 25°C are presented in Figure 9. Each spectrum is a current response as a function of frequency. The calculated corrosion kinetic parameters at different concentrations of the the investigated compounds in 1 M HCl at 25°C (icorr, βa, βc, CF-2, CF-3 and η%) are given in Table 7. From this Table, the corrosion current densities decreased by increasing the concentration of investigated compounds, the inhibition efficiencies increase by increasing investigated compounds concentrations. The causality factors CF-2 and CF-3 in Table 7 are very close to theoretical values that according to EFM theory [39] should guarantee the validity of Tafel slopes and corrosion current densities. Values of causality factors (Table 7) indicate that the measured data are of good quality. The standard values for CF-2 and CF-3 are 2 and 3, respectively. The causality factor is calculated from the frequency spectrum of the current response. If the causality factors are approximately equal to the predicted values of 2 and 3, there is a causal relationship between the perturbation signal and the response signal .Then the data are assumed to be reliable [12]. When CF-2 and CF-3 are in the range 0-2 and 0-3, respectively, then the EFM data is valid. The deviation of causality factors from their ideal values might due to that the perturbation amplitude was too small or that the resolution of the frequency spectrum is not high enough also another possible explanation that the inhibitor is not performing very well [13]. |
| 3.7 Mechanism of Corrosion inhibition |
| From the observations drawn from the different methods, corrosion inhibition of C-steel in acid chloride solution by the investigated inhibitors as indicated from weight loss, potentiodynamic polarization, EIS and EFM techniques was found to depend on the concentration and the nature of the inhibitor. The adsorption of organic molecules on the solid surfaces cannot be considered only as purely physical or as purely chemical adsorption phenomenon. In addition to the chemical adsorption, inhibitor molecules can also be adsorbed on the steel surface via electrostatic interaction between the charged metal surface and charged inhibitor molecule if it is possible. The free energy of adsorption value is not very greater than -40 kJ mol-1 should indicate contribution of physical adsorption. If the contribution of electrostatic interactions takes place, the following adsorption process can additionally be discussed. The investigated compounds have basic character and expected to be protonated in equilibrium with the corresponding neutral form in strong acid solutions. Because Csteel surface carried positive charge, Cl- ions should be first adsorbed onto the positively charged metal surface. Then the inhibitor molecules adsorb through electrostatic interactions between the negatively charged metal surface and positively charged inhibitor molecule and form a protective layer. In this way, the oxidation reaction of Fe can be prevented [40]. The protonated inhibitors molecules are also adsorbed at cathodic sites of metal in competition with hydrogen ions. The adsorption of protonated inhibitors molecules reduces the rate of hydrogen evolution reaction [41]. |
| The order of increased inhibition efficiency for investigated derivatives is: 3 > 2 > 1 as indicated from the different methods. Compound (3) is the most efficient inhibitor. Exhibit excellent inhibition power due to: (i) The presence of two N and three O atoms which are electron denoting atoms which increase the electron charge density on the molecule and (ii) these atoms may add an additional active centers. Compound (2) comes after compound (3) in inhibition efficiency due to the presence of two N and two O atoms with less one O atom than in compound (3). This will decrease the charge density on the molecules and hence, the inhibition efficiency decreased. Compound (1) is the least efficient inhibitor, in spite of the presence of two N and two O atoms. This is due to the presence of Cl atom which is very withdrawing group. |
CONCLUSIONS |
| From the overall experimental results the following conclusions can be deduced: 1. (Z)-N'-(1-phenylethylidene)benzohydrazide derivatives are good inhibitors and act as mixed type but mainly act as anodic inhibitors for carbon steel corrosion in 1 M HCl solution. 2. The results obtained from all electrochemical measurements showed that the inhibiting action increases with the inhibitors concentration and decreases with the increasing in temperature. 3. Double layer capacitances decrease with respect to blank solution when PEBH is added. This fact confirms the adsorption of the investigated compounds molecules on the carbon steel surface. 4. The adsorption of PEBH on the carbon steel surface at different temperature was found to obey the Langmuir adsorption isotherm and this adsorption is physosorption 5. The values of inhibition efficiencies obtained from the different independent quantitative techniques used show the validity of the results. |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
References |
|