ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
YB Sonawane1, MR Shindikar2, MY Khaladkar3*
|
| Corresponding Author: MY Khaladkar, |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Pyrolysis of high density polyethylene (HDPE) waste in the form of carrybags was carried out in an inhouse fabricated glass reactor of one litre capacity. For reaction, waste material of about 200-300 grams was used. An inert atmosphere was created in the reactor by purging nitrogen gas. The maximum temperature in a reactor was kept about 5500C. Reaction was carried out with and without using natural zeolite (NZ) and alumina catalysts. It was found that, time required for completion of the pyrolysis process was 3.5 hrs without catalyst and 2.5 hrs with catalysts. Upon comparison between fuel obtained with and without catalysts, it is observed that in absence of catalyst, process gives about 60-62% yield, with 5 % natural zeolite yield is 65-67 % and with 5 % alumina catalyst it is about 70-71 %. An increase in the yield % and calorific value is observed with both the catalysts but higher effect is seen in case of oil obtained with alumina. Analysis of oil samples by GC-MS showed presence of gasoline, kerosene and diesel fractions (C5-C20) with other high molecular weight fractions from C20-C37 for all systems. Currently there are mechanical systems available for recycling of plastic waste but these systems involve more cost in transportation than recycling. Scaling up of the present method would substantially reduce the transportation cost and would also help in the quest of exploring economically viable recycling system for plastic waste
Keywords |
| Thermoplastic waste, Pyrolysis, Catalyst, Calorific value, GC-MS. |
INTRODUCTION |
| Majority of plastics that are used today are non biodegradable in nature, they remain in environment for long period which affects environmental quality. In India, plastic waste accounts to be about ten thousand tons per day of generated municipal solid waste [1]. As there is no effective segregation and recycling system for these plastic waste generated, it increases load on landfill sites because of their non-biodegradable nature and ultimately causes environmental problems like air, soil and ground water pollution as well as loss of marine biodiversity. Most of the household plastic waste generated in India is of thermoplastic type which includes mainly polyethylene terephthalate, high density polyethylene, polyvinyl chloride, low density polyethylene, polypropylene and polystyrene. HDPE is one of the major components in a plastic waste stream and generally used for making carry bags, plastic bottles and milk and food containers etc. Recycling of HDPE waste is done by different methods like mechanical recycling, chemical recycling etc. Mechanical recycling refers to operations that aim to recover plastics waste via mechanical processes (grinding, washing, separating, drying, re-granulating and compounding), thus producing recyclates that can be converted into new plastics products, often substituting virgin plastics. But major drawbacks of this process are requirement of clean source of post-consumer plastics, the need for efficient separation technology to obtain generically pure resin types and labour-intensive process. Pyrolysis is one of the best methods to minimize burden on landfills. It also generates diesel grade fuel which can be used as a substitute fuel for trucks, boilers and generators in industries. |
| Pyrolysis of HDPE has been studied by Kumar et al. and Ammar et al. [2,3] Studies on pyrolysis of LDPE were done by Sarker et al. and Ademiluyi et al. [4,5]. Obali et al. and Nishino et al. [6,7] used Polypropylene material for pyrolysis without using catalyst. Different catalysts also have been studied in pyrolysis process for enhancing yield of product by Tiwari et al., Osueke et al., Jeong et al. and Miskolczi et al.[8,9,10,11]. There are very few commercial plastic waste pyrolysis plants in India and they have large intake capacity. Plastic waste generated is required to be transported to the larger scale single processing unit which involves high cost of transportation. For running processing unit, high electricity and skilled manpower is required which again increases cost. So, though the process converts waste plastic into valuable product, the total efficiency in terms of cost and energy cannot be achieved. Present work focuses on development of onsite plastic waste processing unit which can be situated at different locations ultimately reduces transportation cost. With the help of this, a major issue of plastic waste disposal can be tackled. |
MATERIALS AND METHODS |
| In present work, experiments were carried out with and without using natural zeolite (NZ) and alumina catalysts where the experiment without catalyst was kept as a control. Feedstock used for the process was HDPE milk carry bags, obtained from household sources. The washing, drying and shredding of material was manually done. The amount of waste material used for each process was about 200-300 grams which can be up scaled upto 1 kg. As a starting experiment comparison was done between no catalyst and with 5% catalysts. Natural zeolite and Alumina were used as catalysts. In two separate experiments 5 % zeolite and 5 % alumina were used as catalysts. The whole set up consisted of nitrogen gas cylinder, heating assembly, an in house fabricated glass reactor with recovery bend, condenser and collection flask. Shredded plastic waste material was fed into a reactor for pyrolysis. Before starting reaction, nitrogen gas with pressure of 5kgf/cm2 was purged for 10 seconds into a reactor to create an inert atmosphere. The experiment was carried out at maximum temperature of 5500C. The vapours coming out after melting of the waste were passed through condenser and liquid obtained was collected in a flask. Uncondensed gases produced during the experiment were passed in cold water bath. |
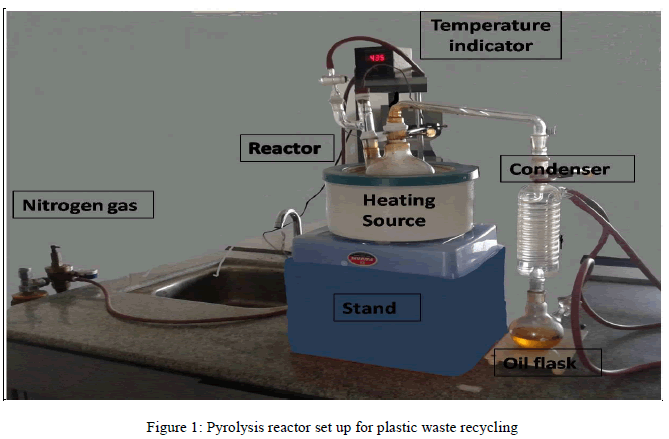 |
EXPERIMENTAL RESULTS |
| Melting point of HDPE was observed about 1300C. The temperature range 450- 5500C gave maximum yield and at a temperature about 5000C, the rate of oil obtained was very fast. Without using catalyst, half an hour was required to start receiving distillate whereas by using catalysts 20 minutes were required. Time required for completion of the pyrolysis process was 3.5 hrs without catalyst and 2.5 hrs by using natural zeolite and alumina. |
| Precautions: While arranging glass reactor set up, all joints should be sealed with Teflon tape to avoid leakage of gases. Nitrogen gas should be purged slowly. Excess pressure of nitrogen gas causes breaking of glass apparatus. Quantity of waste taken should be proportional to the size of reactor; otherwise it will cause obstruction to the generated vapours to get condensed which will result in splashing of the boiling liquid from reactor to collection flask. After completion of process, waxy residue obtained should be kept for cooling in absence of air, otherwise it will catch fire. Weight of oil obtained by the process was calculated by multiplying density of oil with ml of oil obtained. Quantity of oil was recorded in grams and oil percent obtained was also calculated by using formula |
| Product Characterization: |
| Density and specific gravity of oil samples were determined with specific gravity bottle. Calorific value of oil samples was determined by digital bomb calorimeter with make model RSB-3 by Rico Scientific. GC make model Agilent-7890, with FID detector was used and MS make model Jeol Accu TOF GCV with mass range 10-2000 amu and mass resolution 6000 was used. Results showed presence of gasoline, kerosene and diesel fractions with some higher molecular weight fractions. |
| Table 1 gives comparative account of yield percent of oil and wax obtained for HDPE without catalyst and with catalysts and reaction time required for the process. |
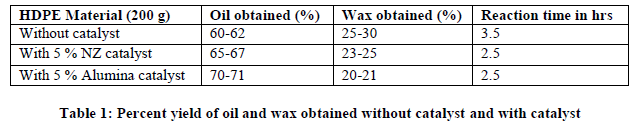 |
| Without using catalyst, oil obtained is minimum ie. 60-62% and by using 5 % alumina catalyst, oil obtained is maximum i.e. 70-71%. By using catalysts, time required for completion of process is reduced by 1 hr. As compared to natural zeolite, alumina is better catalyst in terms of yield and physical appearance. Result shows that increase in oil quantity ultimately decreases wax quantity which is desirable. |
| Table 2 displays data about Specific gravity and calorific value of oil samples with and without catalysts. |
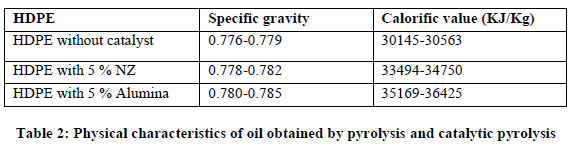 |
| There is gradual increase in Specific gravity of the oil samples with catalysts as compared to oil samples without catalysts which shows increase in calorific value respectively. High calorific value of oil with alumina and natural zeolite shows its usefulness as a fuel for combustion. |
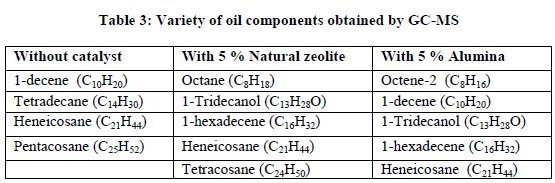 |
| (Gasoline fractions: C5-C10 , Kerosene fractions: C11-C13, Diesel fractions: C13-C18) From GC-MS plots, it is observed that pyrolysis product obtained without catalyst and with catalysts contains series of aliphatic hydrocarbons from carbon numbers C4 to C37. |
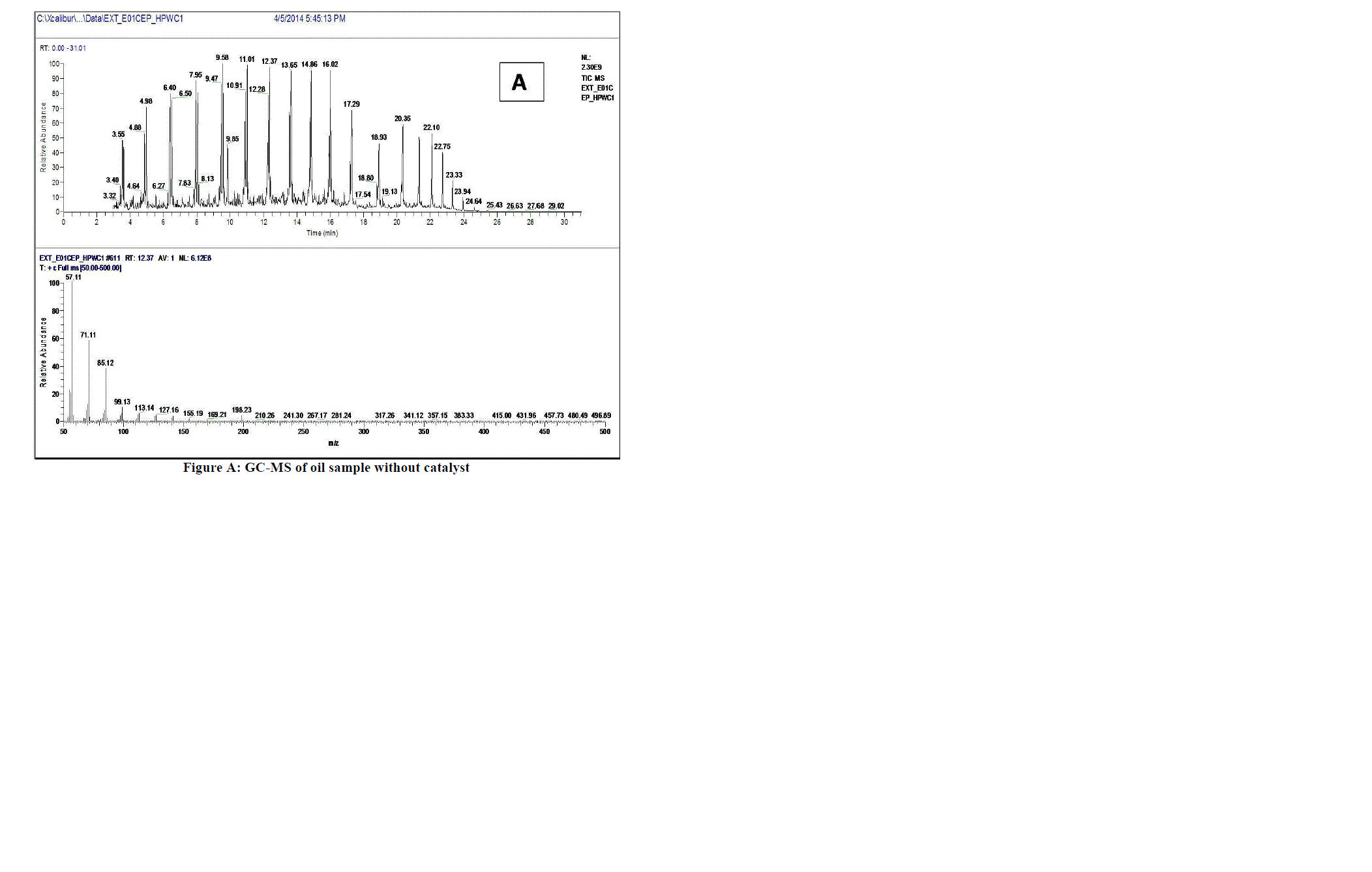 |
| Fig. A shows GC-MS plot of oil obtained without catalyst which gives some low molecular weight alkanes, alkenes and some high molecular weight fractions like Heneicosane, Tetracosane etc. |
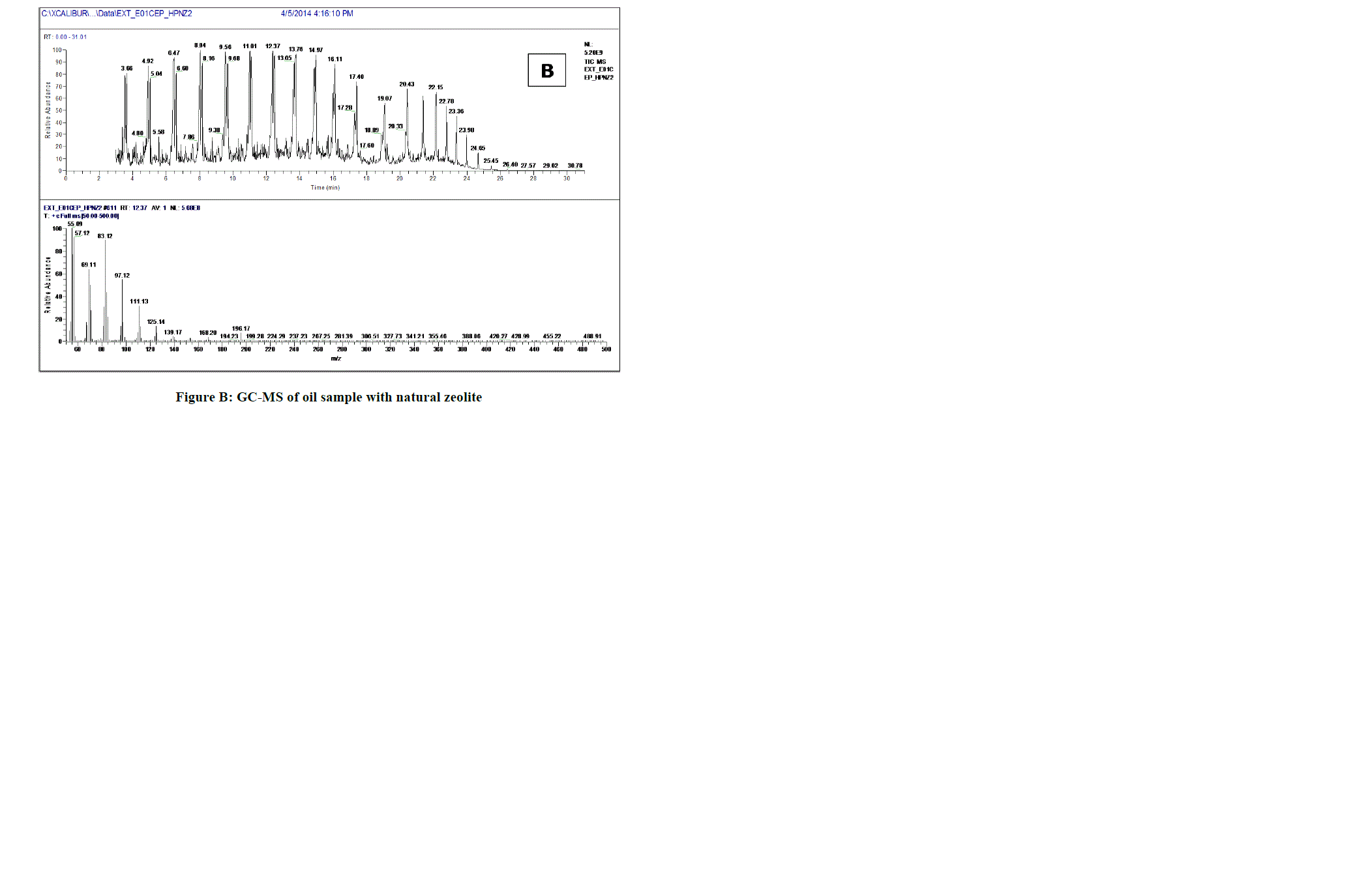 |
| Fig. B. shows GC-MS plot of oil sample obtained by using natural zeolite, range of gasoline fractions obtained is more than that of without catalyst but range of high molecular weight fractions is also more which is undesirable. |
 |
| Fig. C shows GC-MS plot of oil product obtained with alumina. It has wide range of hydrocarbons (C4-C21) in which higher fractions like tetracosane, pentacosane are absent which seems to be desirable. From comparative study of A, B and C graphs of GC-MS, it is observed that oil obtained with 5 % alumina gives more variety of hydrocarbon products which include gasoline(C5-C10), kerosene (C11-C13) diesel (C13-C18) and some waxy fractions (C20-C37). |
CONCLUSIONS |
| Pyrolysis of HDPE waste used in the form of carrybags gives yield in the form of liquid fuel, gas and waxy residue. In pyrolysis, the temperature range 450- 5500C gives maximum percent of oil and less yield of wax. At a temperature about 5000C, the rate of oil obtained is very fast. By using catalyst in pyrolysis process, the reaction time can be minimized upto an hour for 200-300 gm of plastic waste. The percent of oil can be increased and wax quantity can be reduced with the help of catalyst. The oil obtained by catalytic pyrolysis has higher calorific value (33494 to 36425KJ/Kg) as compared to pyrolysis without catalyst (30145-30563KJ/Kg). After studying pyrolysis by using natural zeolite and alumina catalysts, it is concluded that as yield obtained with alumina is higher than that of natural zeolite and calorific value determined with alumina is also higher so alumina is better catalyst. The oil obtained without catalyst and with alumina and natural zeolite has hydrocarbon fractions from C4 to C37. Oil obtained with alumina has wide range of hydrocarbons and shows presence of gasoline, kerosene and diesel fractions. |
References |
|