ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Akhilesh Prabhakar1, Praseetha P Nair2, K E George3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Polystyrene based nanocomposite was prepared by adding Kaolinite clay and polybutadiene rubber to polystyrene, by using insitu polymerisation technique. The nanocomposite so formed were characterised by TGA and DSC analyses. The study shows that thermal stability of polystyrene can be improved by the addition of nanoclay. When nanoclay is added, a high performance high impact polystyrene can be prepared by addition of a lower amount of PB than the PB content in commercial supply. The optimum composition of clay and rubber content was determined using response surface method in Minitab
Keywords |
| Polymer nanocomposite, kaolinite clay, polystyrene, insitu polymerisation, optimisation of composition |
INTRODUCTION |
| The search for new materials is going on all over the world. Materials are synthesized and analysed for advanced properties. Polymers form the most advanced and adoptable class of engineering material available today. They yield a wide range of properties. The importance of polymers over other available materials is that their properties can be modified by mixing with other polymers [1] [2] or by adding modifiers like fillers. The blend of polystyrene with polybutadiene rubber is known as HIPS (High Impact Polystyrene)[3]. The graft polymer so formed having high mechanical strength [4]. Use of nanomaterials for the modification is the latest development. A small amount of nanomaterial can alter the properties of the material [5]. Modifying polymers with nanofillers yield the ultimate improvement in properties hence polymer nanocomposite is a widely studied research area. Nanomaterials like graphite [6], CNT [7], Montmorillonite clay [8], Laponite clay [9] etc are used. Montmorillonite was the initially used nanoclay for synthesis of polystyrene nanocomposite. Due to its high production cost more studies should be conducted using with clays like Kaolinite. Clays are hydrophilic in nature. They are modified using vinyl or amino groups [10]. In this work Kaolinite clay (vinyl modified) was used . |
| Synthesis method varies as solution intercalation [9], miniemulsion [11], micro emulsion [12], bulk method [13] and insitu polymerisation [6, 7, 14]. Melt intercalation is the commonly used method for modification using nanofiller. It has a disadvantage of improper dispersion. Better dispersion can be obtained by insitu polymerisation. It can impart only partial dispersion and result in a composite with nonuniform properties. So insitu polymerisation technique is employed in this study. |
| The combined effect of rubber and clay can not be predicted by analyzing sample containing only clay or rubber. A nuber of combinations is to be synthesized and analysed. It can be made simple by using statistical tools. Response surface methodology (RSM) consists of a group of empirical techniques devoted to the evaluation of relations existing between a cluster of controlled experimental factors and the measured responses according to one or more criteria [10]. The analysis was done with statistical software, Minitab. The effects of 2 variables were studied using RSM. Box Behnkon and central composite were two response methods available in Minitab. The most common experimental design used in RSM is the central composite design (CCD), which has equal predictability in all directions from the center. The response of the system was expressed by the following second degree polynomial equation: |
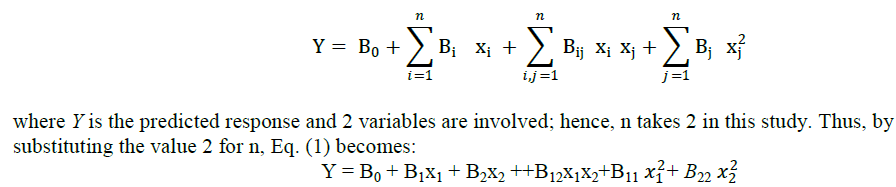 |
II. EXPERIMENTAL |
A. Materials |
| Styrene( S-0800, Rolex Chemicals) |
| Nanoclay (Nanocaliber 100 V, M/s English Clay Ltd. Vely, Trivandrum, Kerala.) |
| BPO (Analar grade) |
| NaOH (Analar grade) |
| Polybutadiene rubber (Cisamer 1220 ) |
Synthesis |
| Washing of styrene monomer is important as it contains inhibitors. Inhibitors are added to the styrene monomer in order to prevent polymerisation during storage. 5% NaOH was used for washing purpose. 25ml styrene is taken in a separating funnel. Equal amount of 5% NaOH is added to it. It is shaken well. Allowed to settle, NaOH layer is separated out. Same procedure is repeated 3times with DM water and 3times with NaOH solutions alternatively. One more final rinse is done with DM water. Moisture present in styrene can affect polymerisation. Even then it is to be removed. For this pellets of molecular sieves are used. Molecular sieve pellets are added to the styrene for 15 minutes. They absorb the water drops if any. The pellets can be removed by decantation. |
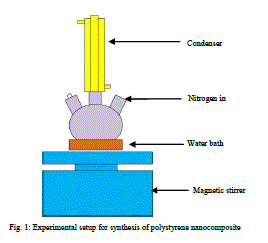
1) Experimental setup |
| The experiment was done in a three neck flask. It was heated to 900C in water bath on a magnetic stirrer with continuous stirring. A condenser is connected to the 3 neck flask. It is provided to trap the escaping vapours of styrene. It is covered at the top using a piece of cotton. N2 is passed through the reactor when the reaction is carried out. This will ensure an inert atmosphere for the polymerisation reaction and avoid any external factors affecting the reaction. |
 |
2) Polymerisation |
| Washed styrene was transferred to the three neck flask. Water circulation was started through the condenser. Heating is provided to increase the temperature to 800C. Nitrogen gas was introduced to create an inert atmosphere above the monomer surface. Weighed amount of rubber is added when the temperature become steady. 30 minutes time is given for dissolving rubber. When the solution becomes homogeneous, benzoyl peroxide was added. Temperature was maintained at 800C, as the BPO become active only at that temperature. Stirring continued for about 25 minutes. Then the clear solution changed to turbid. The viscosity also increased. At this point nanoclay added. A dispersion time of 30 more minutes was given. Whole liquid was transferred to a Teflon mold. Curing time of one week was given. |
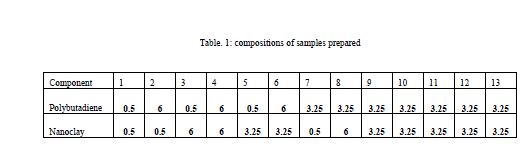
| It was desired to vary the composition from 0.5 to 6 weight percentage. The limits were given to Minitab and design of experiment was done. Samples were prepared at these combinations and analysed. |
 |
C. Analysis |
| TGA analyses of the samples were done using Perkin Elmers STA 6000 apparatus. The shape and morphology of the particles were analysed by scanning electron microscopy (SEM) Joel Model JSM 6390 LV. Exfoliation is ensured by comparing XRD of Kaolinite clay and synthesised composite. |
III. RESULTS AND DISCUSSION |
A. Thermal Gravimetric Analysis (TGA) |
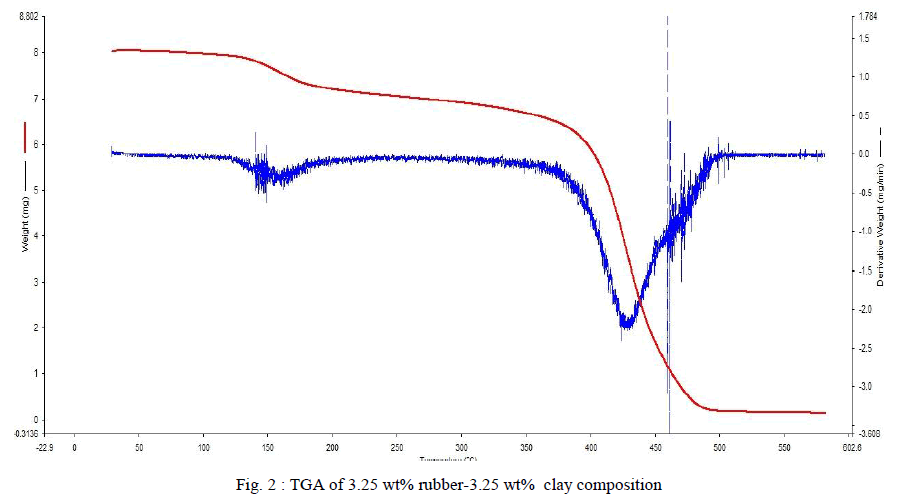
| TGA measures the change in weight of a sample when it is heated, cooled or held at a constant temperature. The change in weight can be obtained as a graph against temperature or time. TGA analysis of the samples was done using 5-10 mgs of sample. Each sample was heated from 200C to 6000C in nitrogen atmosphere. Heating provided 200C per minute. Fig .2 shows TGA result of the sample with 3.25 weight% rubber and 3.25 weight% clay. The red colour curve shows the weight loss per degree of increase in temperature. Initially there were slow but steady decrease in the weight. It is due to the removal of moisture or trapped monomer if any. When the temperature reaches 3800C degradation starts. It reaches its maximum at a temperature of 4400C. It can be determined using the derivative of the weight loss curve provided by the instrument itself .The blue thick line represents the derivative weight loss. |
 |
B. Differential Scanning Calorimetry (DSC) |
| DSC measures the amount of energy absorbed or released by a sample as it is heated, cooled or held at a constant temperature. It is achieved by placing two temperature probes in the furnace and simultaneously measuring the temperature of the sample and furnace temperature while heating the sample at a constant rate. This technique is used for polymer and pharmaceutical applications. The DSC will be resulted in a heat input against temperature curve. Glass transition temperature, crystalisation point, melting point etc can be determined from the DSC curve. |
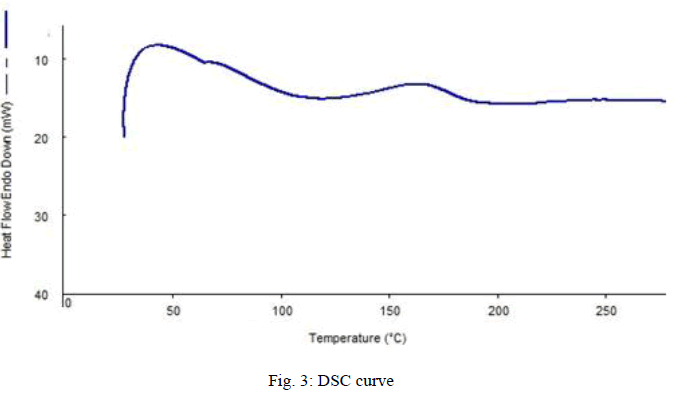 |
| Fig. 3 shows the DSC curve of sample prepared with 3.25 wt% PB and 3.25wt% clay. From the graph, it can be understand that, the glass transition occurs at a temperature of 1440C. It was indicated by the increase in heat flow. It is the glass transition temperature where the sample starts withdrawing heat at increased rate. Then it reaches its melting point, where it melts completely. Then the heat absorption reduces. And continues till its degradation starts. Here we can see the melting point as 1960C. DSC for the rest of the sample also was done. |
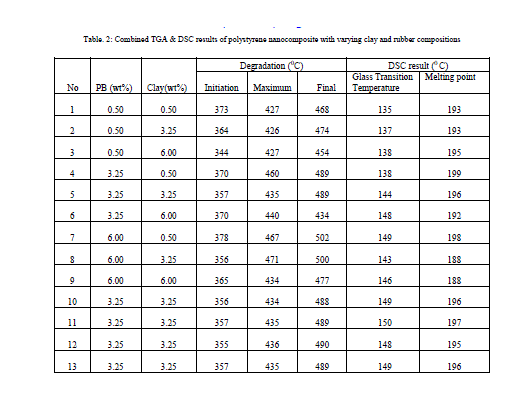 |
D. Response Surface Regression |
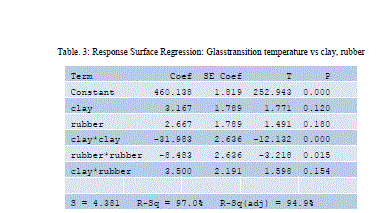 |
| Design equation obtained by regression analysis is given by |
| Glass transition temperature = 128.341+ 3.31585 A+ 6.50043 -0.346537A2-0.743232 B2 -0.0661157 AB |
| Where, |
| A- Clay composition in weight percentage |
| B -rubber composition in weight percentage |
| Considering p values A&B having less effect than its power terms |
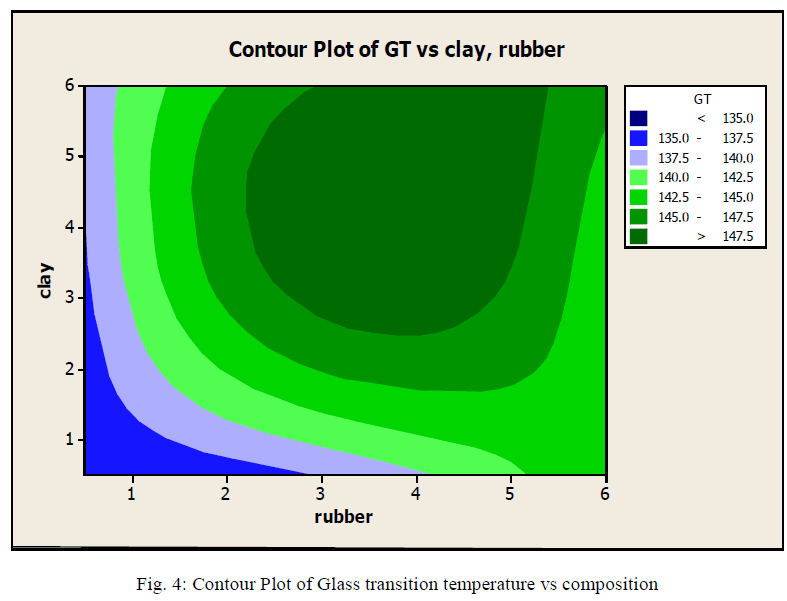 |
| Fig.4: shows the contour plot provided by Minitab showing Glasstransition temperature at different rubber and clay compositions. The different ranges are shown in different colures From the graph we can understand that the cay composition increases glass transition temperature. The result can be compared with what we got in TGA analysis. We can say that the optimum composition is at clay composition of 2 weight % to 5weight % and rubber of 2.5 to 5 weight %. It holds the range we got in TGA analysis. |
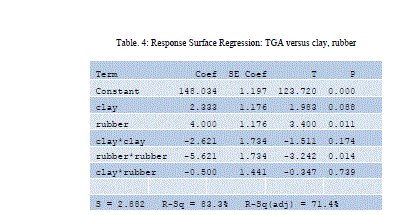 |
| Design Equation obtained using Minitab |
| TGA = 401.614 + 27.1367 A + 6.75653 B - 4.22913 A2 - 1.12169 B2 + 0.462810 A*B |
| Where, |
| A Clay composition in weight percentage |
| B rubber composition in weight percentage |
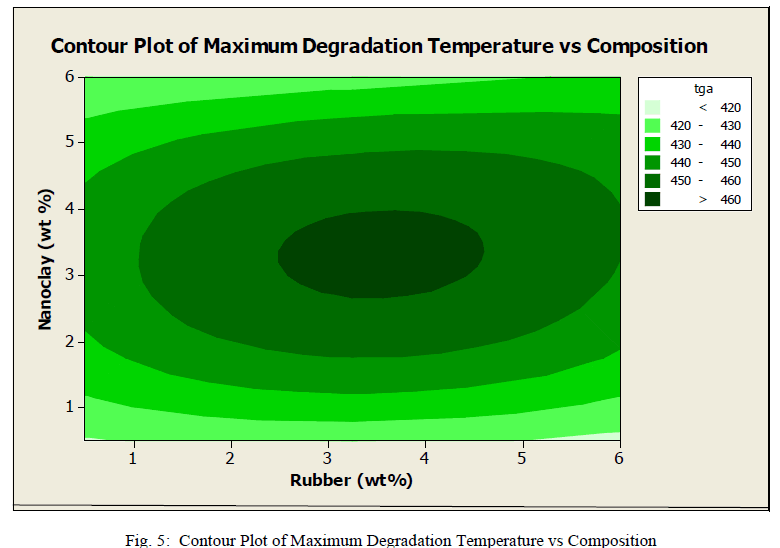 |
| Graph shows the contour plot provided by Minitab showing degradation temperature (maximum) at different rubber and clay compositions. The different ranges are shown in different colures. It is desired to have higher degradation temperature. The inner circle shows the region where, maximum degradation temperature is obtained. In that region clay composition ranges from 2.5 weight% to 4.5 weight% and rubber 2.5 weight% to 4 weight%. So from the analysis it can be understand that the above composition is the optimum composition for thermal stability. The effect of nanoclay is very low at lower compositions. But as the percentage increases, the filers got dispersed and it imparts thermal resistance to the material. If we further increase the clay composition agglomerates will be formed. It will ultimately reduce the strength and affects negatively on composite properties. |
 |
IV. CONCLUSION |
| Study shows that Kaolinite clay can be efficient nanofiller in polystyrene. The insitu method is tends to be attractive in generating polystyrene based nanocomposites. If PB is used along with nanoclay high impact polystyrene can be formed with less amount of rubber. From response surface analysis composite with a composition of 3.25 weight %rubber with 3.25 weight % clay is found to have the optimum composition. |
References |
|