ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Chaman Mehta.C1, M. P. Rajesh2 V.Sridevi *3, Divya M. L4 and Satya Ch.V5
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This work attempts to study and optimize aqueous two-phase system (ATPS) made of PEG, sodium carbonate and PEG, sodium sulphate. Three factors ;PEG concentration, salt concentration and pH affecting the papain partitioning were studied. The statistical analysis showed that for both systems the phase forming salt concentration significantly affect the “K” value for papain partitioning. However, the performance of PEG/Na2CO3 system was generally better than PEG/Na2SO4 system. Hence, detail study was carried out on the PEG/Sodium carbonate system using CCRD (Central Composite Rotatable Design) in Response Surface Methodology (RSM). The optimal condition gave a “K” value of partitioning of 1.51 with a yield of 76%.
Keywords |
| Aqueous two-phase system, Polyethylene glycol, Sodium Carbonate, Central composite design. |
INTRODUCTION |
| Conventional techniques for separation and purification of biomaterials have some disadvantages such as insufficient selectivity, high energy consumption and possibility of denaturation of biomolecules when organic solvents are used in extraction processes. Most of these difficulties can be overcome by conducting the bio-separations in aqueous two-phase systems (ATPS). Aqueous two-phase system was developed in Sweden during mid-1950s for the separation of macromolecules, cells and organelles. These systems were initially applied to the separation of plant organelles and viruses. Since, then attention has been directed towards widening its application scenario. During the last two decades [1] lot of work has been done to develop feasible separation processes using aqueous two-phase systems for various biological materials, proteins and recombinant proteins. |
| The papain present in the latex of papaya (Carica papaya) has been extensively studied and is an enzyme of industrial use and of high research interest [2]. The latex of carica papaya is a rich source of cysteine endopeptidases, Glycyl endopeptidases, Chymopapain, Papain and Caricain, which constitute more than 80% of the whole enzyme fraction. This enzyme is widely used as meat tenderizer, and has also several other applications for e.g, Shrink proofing of wool, treatment of edemas, purification of papain from papaya latex has involved the use of various chromatographic techniques which includes Ion-exchange and Gel filtration [3]. ATPS has shown interest especially in the view of providing integrated clarification, concentration and purification of the target product in one unit operation [4]. They also have been applied successfully for large scale enzyme separation and purification [5] use of PEG-Phosphate system has earlier been reported for separation and purification of papain from papaya latex [6]. The aim of this study is to investigate for performance of aqueous two-phase system and the significant factors that may affect papain partitioning of two different systems. Response surface methodology [7]will be used to identify the significant factors and develop model to predict the response of the ATPS and obtain the optimal conditions for papain partitioning [8]. |
II. MATERIALS AND METHODS |
Materials |
| Polyethylene glycol with Molecular weights 4000 and 6000, sodium sulfate, and sodium carbonate were obtained from Himedia. Sodium chloride was obtained from Sisco (Chennai). Crude papain was purchased from Shri Ganesh industrial enzymes (Madhya Pradesh). All other chemicals were of analytical grade. |
Native PAGE |
| Non-denaturing electrophoresis was carried out by the method of [9] with some modification, using a gel consisting of a resolving gel (pH 4.3, 15%, w/v, and acrylamide) and a stacking gel of 4% acrylamide (pH 6.7). The upper and lower chamber electrode buffer (pH 4.5) consisted of 100mM Glycine –0.14M acetic acid. The protein sample was diluted (1:1, v/v) with stacking buffer (pH 6.7) containing 10% sucrose and bromophenol blue (used as a tracking dye) prior to loading onto the gel. Electrophoresis was run at a constant current of 40 mA [10]. The protein samples migrated towards the cathode during electrophoresis. The presence of other proteins (chymopapain, Glycyl endopeptidase) in the crude extract of papain (after staining with Coomassie Brilliant Blue R-250. The in situ proteolytic activity was determined on the gel after cathodic electrophoresis (Fig. 1). The gel was rinsed twice with 50 ml of 0.1M Tris–HCl buffer, pH 8.0, and then a solution containing 0.5% (w/v) agarose and 1.8% (w/v) casein in the same buffer was applied on the gel surface. The gel was incubated at 37 0C between 12 and 48 h to visualize the clear zone of proteolytic activity. |
2D Gel Electrophoresis |
| The 2D-GE utilizes the isoelectric point (PI) of iso-electric focusing (IEF) [11] in the horizontal dimension and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) in the vertical dimension [12]. Using a scanner to scan the 2D-GE, a 2D-GE image can be obtained. The spots on the gel shows the proteins present in the crude sample and then doing analysis papain can be separated. Detection of spots on the gel was done with staining the gel with coomassie brilliant blue. Three spots were obtained on the gel with pI close to 10 (Fig.1 ). 2D analysis of crude papain showed that two or three contaminating proteins are present in crude along with papain. |
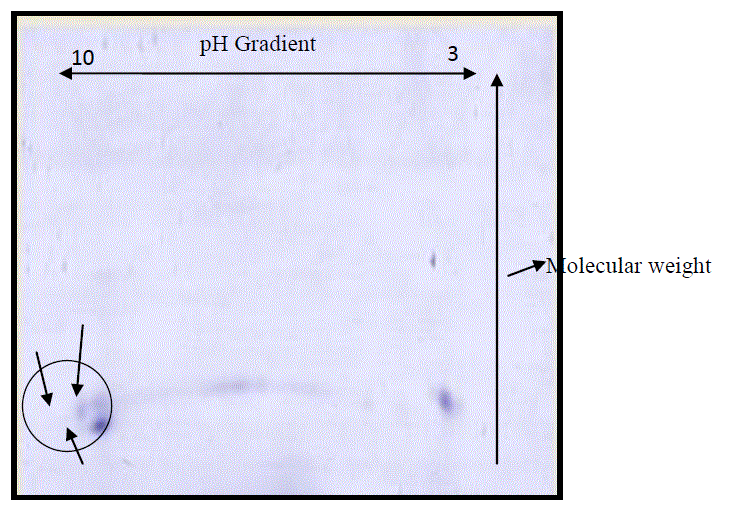 |
| Fig. 1 Coomassie brilliant blue-R250 stained 2-D gel strip. The protein sample was first subjected to isoelectric focusing with a pH 3 to 10 strip for 16 h followed by SDS-PAGE for 8 h. |
Chromatography of Crude Papain |
| Aliquots of proteins dissolved in 100mM Glycine -NaOH buffer, pH 10.6, were applied to a DEAE cellulose column (1.5 x 20 cm) equilibrated with the same buffer [13]. The material was eluted using a continuous gradient (0.4 to 1.0 M) of Glycine-NaOH buffer, pH 10.6. The peaks obtained were delimited (Fig. 2), pooled and eluted with 1M NaCl buffer. Initial no of proteins are given in the form of peaks to know and purify the protein (Papain) of interest. |
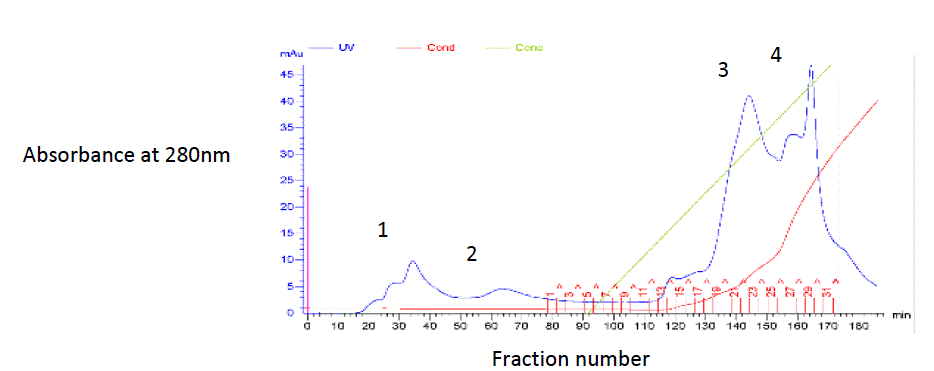 |
| Fig. 2 Chromatogram of crude papain. The column (1.5 x 10 cm) after equilibrated with 100mM Glycine-NaOH buffer, pH 10.6. 20mg of protein is added and eluted with 0.4, 0.6, 0.8 and 1.0 M with the same buffer at a flow rate of 30 mL/h. |
Determination of Protein Content |
| The protein content in the samples during purification was determined by Bradford method [14]. |
Purification of Papain Extraction in Aqueous Two-Phase System |
| The crude papain was mixed with water and pH adjusted using 6M phosphate buffer. For extraction in aqueous two-phase system, defined amounts of solid PEG and sodium carbonate were added to the Crude preparation (30 g)[15], and the total mixture was made up to 50 g with water. The mixture was then gently shaken for 15 min. The two phases were separated by centrifugation at 9000×g for 30 min at 40C [16]. Aliquots of the phases were taken for determination of protein concentration and protease activity. For calculation of protease activity in the two phases, volumes of the respective phases were taken into consideration [17]. The presence of papain was verified by cathodic gel electrophoresis and FPLC. The dialyzed solution was subjected to ion-exchange chromatography for separation of the enzyme from PEG[18]. The solution was loaded on a DEAE -cellulose column (1.5 cm×2 cm) equilibrated with 100mM Glycine-NaOH buffer, pH 10.6 and after washing the enzyme was eluted from the column with the buffer containing 1M NaCl [19]. Papain was largely eluted at the front of the two buffers. |
Enzymatic Assay |
| The proteolytic activity was determined according to the procedure of Tyrosine unit method with slight modification. The reaction mixture contained 200μl of 50mM cysteine–20mM EDTA (disodium salt), pH 8.0, 700μl 50mMTris–HCl buffer, pH 10.0 and 100μl enzyme solution [20]. The mixture was incubated at 37oC for 5 min before starting the reaction by adding 1ml of 1% (w/v) casein solution. After 10 min, the reaction was stopped by adding 3ml of 5% (v/v) tri chloro acetic acid (TCA) and then cooled for 1 h. The reaction mixture was centrifuged, and absorbance of the supernatant was measured at 275 nm. The reading was corrected for a blank in which the enzyme was added after addition of TCA. |
Experimental design Two -level fractional factorial design |
| A factorial design is experiments which in each complete trial or replication of the experiment, all possible combinations of the levels of the factors are investigated. For any experiments which have k factors, each at only two levels (i.e. high (+1) and low (−1)), they are known as 2 level factorial design (2k) [21]. The number of experimental run required to complete a replicate is given by 2×2× . . . ×2=2k where k is the number of factor, thus giving it its name. If it can be reasonably assumed that certain high order interactions in the system is negligible, then information on the main effects and low order interactions may be obtained by running only a fraction of the complete factorial experiment [22]. Running just a partial number of a complete experimental design is known as fractional factorial design [23]. An important feature in a fractional factorial design is aliasing of the factors. The degree of aliasing present in the factors is given by the design resolution.PEG/sodium carbonate and PEG/sodium sulfate systems were initially investigated with the aid of two-level fractional factorial. The five factors which were considered to affect the protein partition coefficient in the ATPS systems were the PEG concentration of concentration of phase-forming salt, concentration of sodium chloride and Ph [24]. The response measured was the papain partition coefficients shows the experimental conditions for each system studied. The factors which are identified as important or significant are then investigated more thoroughly in subsequent experiment. Response surface methodology (RSM) The basis of RSM is form of an experimental design and the most frequently used is the central composite rotatable design (CCRD) for the prediction and verification of model equation as well as the optimization of the response as the function of the independent parameters [23]. CCRD experimental design is preferred and widely used for fitting a second order models. |
III. RESULTS AND DISCUSSION |
| As papain is a protease of broad specificity and no specific synthetic substrate is available, casein was used as a substrate to determine the total protease activity present in the latex while the purity of papain was determined by electrophoresis and chromatography. On cathodic gel electrophoresis, the proteins in the latex separated as three bands. One of these proteins was identified as papain according to its mobility that was equal to that of the standard papain and its in situ proteolytic activity on polyacrylamide gel. The electrophoresis analysis of the latex protein also indicated that papain constituted about 8% of the total protease (Fig.3). The other component in the crude papain includes chymopapain, glycyl endopeptidase. |
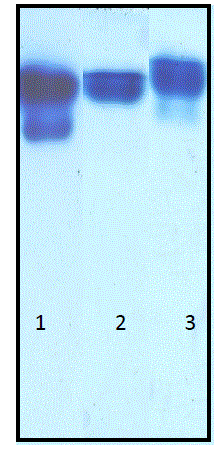 |
| Fig. 3 Non-denaturing polyacrylamide gel electrophoresis using 15% acrylamide gel and glycine buffer, pH 4.3. Staining was by Coomassie Blue R-250. Lane 1: Crude papain (90μg) Lane 2: Standard papain (40μg) Lane 3: Aqueous two-phase extracted sample. |
Purification of Papain by Aqueous Two-Phase Extraction |
| As sodium carbonate is commonly used for separation of cysteine proteases, a two-phase system composed of PEG–sodium carbonate was evaluated for partitioning of papain. According to the phase diagram of PEG–Na2CO3 the system comprising 20% (w/w) PEG 4000–14% (w/w) Na2CO3 provides two separated phases. The system containing more than 20% (w/w) PEG resulted in a highly viscous mixture, whereas the system consisting of more than 14% sodium carbonate provoked the precipitation of protein. Studies on partitioning of total protease activity and of papain by varying different parameters (initial protein concentration, pH, concentration of phase components) showed that extremely low levels of total protease activity were recovered in the PEG rich upper phase, which contained the major fraction of papain present in the crude extract. This may reflect a higher salting out effect of sodium carbonate on papain owing to its higher surface hydrophobicity as compared to the other proteases. Consequently, papain is preferentially partitioned to the top phase. This is also in accordance with the relatively higher papain recovery the other hand; PEG concentration did not have any significant effect on protease partitioning. Partitioning at protein concentrations ranging between 10 and 40 mg protein/ml in 20% PEG–14% Na2CO3 revealed no notable variation in protease partition to the top phase. A significant decrease in partition was indeed observed at higher protein concentrations (60 mg/ml and above) probably due to the limiting solvating effect of PEG. Highest recovery of protease (including papain) activity was achieved at pH 9-10; lowering the pH had a more negative effect than increase in pH, and was attributed more to the inactivation of the proteases than any direct effect on partition. According to electrophoresis results, all the papain in the crude was moved into PEG phase which represented ∼8% of the total proteolytic activity in the crude papain. All the PEG-Na2CO3 systems except the system of 20% PEG–14% Na2CO3 provided pure papain in the top phase. Interference with chymopapain was thus not noticed, as was the case in PEG– carbonate system. The remaining proteolytic activity corresponding to chymopapain, glycyl endopeptidase and caricain remained in the salt rich (bottom) phase. The optimal conditions for extraction turned out to be a two-phase system composed of 20% (w/w) PEG–14% (w/w) Na2CO3 and crude papain containing 20–40 mg protein/ml at pH-9.Furthermore, the pure papain was obtained in a more concentrated form since the top phase volume was about 1/4th the volume of the whole system (top phase volume=5ml; bottom phase volume = 25 ml). The optimal conditions for the papain partitioning were obtained. Optimization of Papain Partition Coefficient The CCRD was able to function as optimal design for the desired response of the system based on the model obtained and the input criteria. The optimization of papain extraction via ATPS was optimized based on the minimum usage of PEG 4000 and sodium carbonate and sodium chloride concentration was in the range of experimentation. The system was maintained with buffer at pH 9. The results from the software indicated that optimized condition for papain partitioning (Fig.4,5,6) was when the PEG, sodium carbonate concentration were at 20.00% (w/w), 14% (w/w) with papain partitioning value of 1.51(K). The experimental runs gave an average experimental value of papain partitioning, log K of 1.483 with the papain yield of 76% and the results were shown in Tables 1&2. |
| Table I Experimental Design matrix for PEG/Sodium Carbonate system using CCRD |
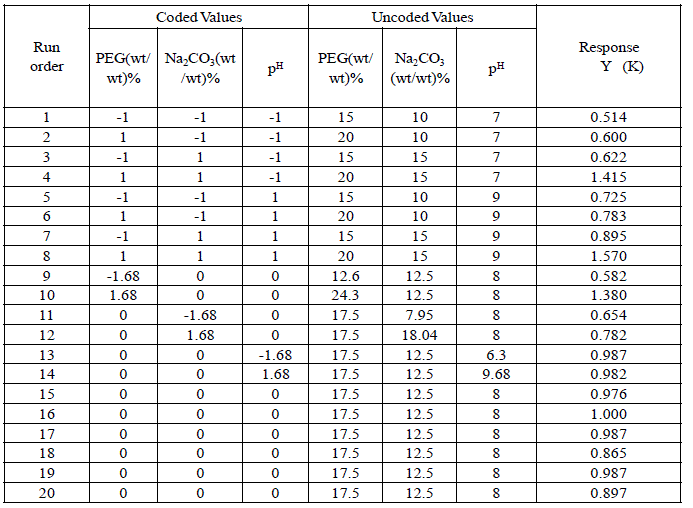 |
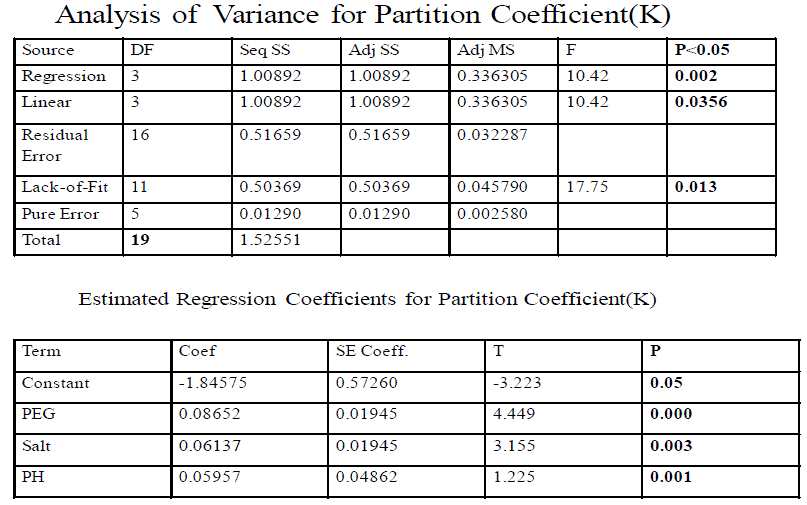 |
| Table II Analysis of Variance for Partition Coefficient (K) |
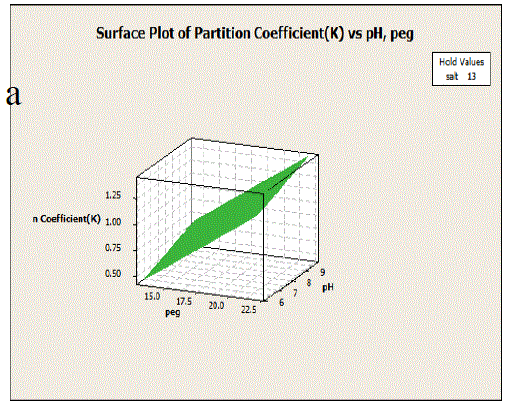 |
| Fig. 4 Response surface curve of papain recovery as a function of pH and PEG (salt concentration was set at central point). |
IV. CONCLUSION |
| The work shows the feasibility of using ATPS as a first step purification of papain using phases composed of PEG/sodium carbonate system. The study demonstrated the PEG concentration, phase-forming salt concentration lead to a higher papain partitioning value.. The CCRD used in this work allowed the definition of appropriate model for the factors studied which in turn lead to the definition of an optimum condition. Papain partition coefficient of 1.51 and a yield of about 76% are undoubtedly a good result for a first step purification. |
ACKNOWLEDGEMENTS |
| We are indebted to Dr. M. P. Rajesh for guidance and also thank Dr. K. Ramasamy for his valuable suggestions. I thank my colleagues Divya and Kalaivani for helping me during my project and finally my college providing the chemicals during my project. |
References |
|