ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Dhanya Sunil 1, Ranjitha C 1, Rama M 1, KSR Pai2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Colorectal cancer is the third most common malignancy in man, with significant morbidity and mortality. Metastasis, the major cause of cancer-associated deaths occurs as a multistep process, where cancer cells detach from the primary tumor, intravasate circulation to disseminate and invade surrounding tissues to form the secondary tumors. The effectiveness of many anticancer drugs is limited by their toxicity to normal rapidly growing cells. Four oxazepines were synthesized by the cycloaddition reaction between schiff bases and maleic anhydride, which were characterized by CHN analysis and advanced spectral techniques. The cytotoxicity of oxazepines against HCT116 (human colon cancer) cell lines were studied using Sulphorhodamine-B (SRB) assay, and their antimigratory properties using wound healing assay. 1-[2-(2,3-dihydro-1H-indol-3-yl)-4,7-dioxo-4,7-dihydro-1,3-oxazepin-3(2H)- yl]thiourea (2b) exhibited very low IC50 in SRB assay with good antimigratory activity as observed in wound healing assay. Snail 1, a transcription regulator of E-cadherin induces epithelial-to mesenchymal transition, reduces intercellular adhesion and increases cell motility, endows epithelial cancer cells with migration and invasive properties. Snail1 is upregulated in several human cancers and is frequently associated with apoptotic resistance, invasiveness, metastases and poor prognosis and it can act as a molecular target in cancer treatment. The docking studies of 2b with the active site of snail1 suggest it to be a potent chemotherapeutic agent in the treatment of colorectal cancer
KEYWORDS |
| Oxazepines, Anticancer, Migration, HCT116, Snail1. |
I. INTRODUCTION |
| Human colon cancer is one of the most commonly diagnosed cancers mainly occurring due to lifestyle and increasing age accounting for 30 % of all cancer deaths. The primary treatment involves surgery followed by chemotherapy. Unfortunately, synthesized anticancer drugs fail to distinguish between malignant cells and normal cells and target the metabolically active cells with an added disadvantage of unpleasant side effects. The development of novel approaches in diagnostic methods and synthesis of new selective anticancer drugs having high efficiency, low toxicity, and minimum side effects by developing new approaches based on the advances in knowledge of cancer biology is the major requirement of the current drug research and development in cancer therapy. |
| Oxazepine is unsaturated non-homologous seven membered heterocycle containing oxygen in position 1 and nitrogen in position 3 in addition to the five carbon atoms. It is prepared by the pericycliccycloaddition of schiff bases with maleic, phthalic, nitrophthalic and succinic anhydrides [1]. Oxazepine derivatives was found to exhibit a vast variety of biological activities like antibacterial [2], antifungal [3], hypnotic muscle relaxant [4], antagonistic [5], antiinflammatory [6], telomerase inhibitors [7] and antiepileptic [8]. Dibenzo[b,f][1,4]oxazepine has been taken up to the design of potent progesterone receptor antagonists, p38 MAP kinase inhibitors, TRPAI ion channel modulators and histone deacetylase inhibitors [9]. Aryl-annelated[1,4]oxazepine is found to be a vital moiety in many psychoactive pharmaceuticals. |
| Snail1 is a repressor of the invasion suppressor gene CDH1, thereby leading to the transcriptional suppression of crucial transmembrane adhesion glycoprotein E-cadherin [10,11]. The loss of E-cadherin expression leads to a phenotypic change in cells, reducing cell-cell contacts as observed in Epithelial-mesenchymal transition (EMT), giving way to invasion, motility, increased apoptotic resistance and tumor proliferation [12]. EMT, which is pivotal in embryonic development for the correct implantation of the embryo and organogenesis, is normally inactive in differentiated somatic cells [13,14]. Downregulation of E-cadherin reactivates EMT; thereby cancer cells gain the ability to invade and metastasize, which is an important event in tumor progression [15]. Snail 1 has recently been shown to revoke the inhibition of the Wnt/beta-Catenin pathway caused by the anti-tumoral compound 1α,25- dihydroxyvitamin D3 [16,17]. Snail 1 is upregulated and frequently associated with invasiveness, metastases and poor prognosis in several human cancers [18-22]. Snail 1 is upregulated (in tumor-stroma interphase) in 60-70 % of colorectal adenoma and colorectal cancers [23]. In a recently published study of stage II colorectal cancer tissues, tumor buds was associated with increased levels of Snail 1 expression as well as a high incidence of metachronous lymph node metastasis [24,25]. The present study encompasses the synthesis, antitumor and atimigratory studies of oxazepines and their docking studies with Snail 1. |
II. MATERIALS AND METHODS |
A. Chemistry |
| Four Schiff bases were prepared by the amino condensation of thiosemicarbazones and aromatic aldehydes. Oxazepines were synthesized by the cycloaddition of schiff bases with maleic anhydride as depicted in scheme-1. |
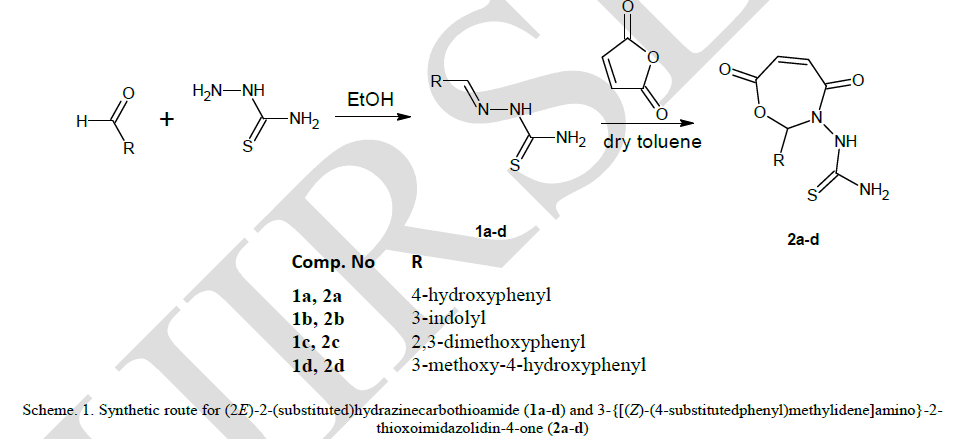 |
| Thin layer chromatography was performed on 0.25 mm silica gel plates using a 3:7 mixture of ethyl acetate and hexane as eluent and identified in UV chamber to monitor the progress of reaction and to check the purity of oxazepines. Melting points were determined by open capillary method and were uncorrected. The elemental analysis of the newly synthesized compounds was carried out in Flash thermo 1112 series CHN analyser. |
B. Anticancer activity |
| All the compounds synthesized were studied for their anticancer properties in HCT116 (colon-carcinoma) cells cultured in Dulbecco’s Modified Eagles Medium (DMEM) with 10 % Fetal Bovine Serum (FBS) in 5 % CO2 atmosphere at 37 °C. |
| Sulphorhodamine-B (SRB) assay: HCT116 cells were seeded at a density of 104 cells/well in 100 μL of medium in 96- well plates and incubated for 24 hours at 37 °C in CO2 atmosphere. Oxazepines were dissolved in 0.2 % DMSO and diluted with media. The cells were then treated with different concentrations (12.5-200 μg/mL; 100 μL/well) of oxazepines. Medium containing 0.2 % DMSO was added to control wells. After 48 h incubation, 50 μL of ice cold 30 % TCA was added to each well, incubated at 4 °C for 1 h and washed with distilled water. Further, 50 μL of 0.05 % w/v (in 1% acetic acid) SRB solution was added, incubated in dark for 30 min, plate rinsed with 1 % acetic acid and dried at room temperature. Finally, 10 mMTris base was added and the absorbance was read at 540 nm on a scanning multi-well plate reader (ELx800, BioTek Instruments Inc., Winooski, VT, USA). The percentage of cell death for SRB assay was calculated using the following formula: |
| % cytotoxicity = [(CA – BA) – (TA – BA)] / (CA – BA) |
| Where, CA, TA and BA are absorbance of control, test (oxazepines) and blank, respectively [26]. |
| Monolayer wound healing assay:Cell migration or motility of HCT116 which is crucial to tissue repair, as well as cancer invasion and metastasis was studied using wound healing assay [27].A wound gap in a cell monolayer is created by scratch, followed by monitoring the “healing” of this gap by cell migrating and growth towards the center of the gap, hereby filling up the “gap”. Cells were seeded in 6-well plates at a density of 1 × 105 cells/well containing DMEM media supplemented with 10 % FBSand incubated till cell growth of 80 % confluence was achieved. The media was aspirated and a single scrape (wound) was created in a linear fashion throughout the center of the plate using a sterile 1 mL micropipette tip to create a denuded zone. Then, cellular debris was removed by washing with phosphate buffer saline (PBS) and the cells were exposed to doxorubicin (1 μg/mL) and 2b (10 μg/mL) in DMEM media. The distance of cell migration (wound closure in μm) were measured using microscope stage micrometer and eye piece micrometer at 45× objective in each well at 24 h and 48 h. The digital images were captured using a CCD camera attached to inverted microscope (Nikon Eclipse TS100) soon after creating the wound and at 24 and 48 h after drug treatment. Analyses were performed in triplicates. The migration distance was calculated by subtracting the width of the injury line (at 24 h or 48 h) from the initial width of the injury line (at 0 h) and expressed in μm. |
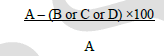 |
| Where A = initial width of the injury line at 0 h; B = width of the injury line in the control; C = width of the injury line at 24 h; D = width of the injury line at 48 h. |
| C. Molecular modeling and docking studies |
| Human Snail 1 crystal structure complexed with FAD with a resolution of 3 Å and corresponding entry code 2Y48 was recovered from the PDB database(www.pdb.org). Surflex dock module of sybylver 1.7 was used ((Tripos Inc. St. Louis, USA)and protomol was generated based on already complexed ligand residues (i.e.FAD) for carrying out docking studies. The proprietary software is licensed to Manipal Institute of Technology, Manipal University, India. The ligands were built using ligand preparation module of surflex descriptors. The best favorable conformation in terms of highest docking score was chosen. The agreement of DMPM to Lipinski’s rule of five was evaluated [28]. |
III. RESULTS AND DISCUSSION |
| FT-IR spectra of oxazepines were recorded in KBr pellet using Shimadzu-8400S spectrometer. The formation of oxazepines were confirmed by the disappearance of a strong absorption due to C=N of schiff bases and 1800-1955 cm-1 for pure maleic anhydride and the presence of a new strong absorption at 1720-1650 cm-1 due to C=O str. The 1HNMRspectrum of oxazepines in DMSO-d6 solvent and TMS as internal standard recorded in Bruker spectrometer showed the aromatic protons around δ 7.4-6.3 ppm and NH and NH2 protons at 9.8 ppm and 2.5 respectively. The mass spectra of oxazepines recorded in a Schimadzu GCMS-QP5050 showed molecular ion peaks which were in accordance with their respective molecular masses. |
| Oxazepine 2b with an indole substituentwas found to be the most potent among all four oxazepines against HCT116 with a very low IC50of 13.2 ± 0.1μg/mL after 48 h incubation. 2a with 4-hydroxyphenyl substituent displayed an IC50 of 32.1 ± 1.7 μg/mL.2c and 2d exhibited relatively less potency with IC5050.4 ± 4.0 and 84.8 ± 2.7 μg/mL respectively. All values are expressed as mean ± SEM (n = 3). Compound 2b which displayed minimum IC50 value was selected to determine the motility of HCT116 cells in in vitro scratch wound healing assay. Fig 1. displays the wound gap after 24 h and 48 h of wound generation for control, standard Doxorubicin (1 μg/mL) and 2b (10 μg/mL). |
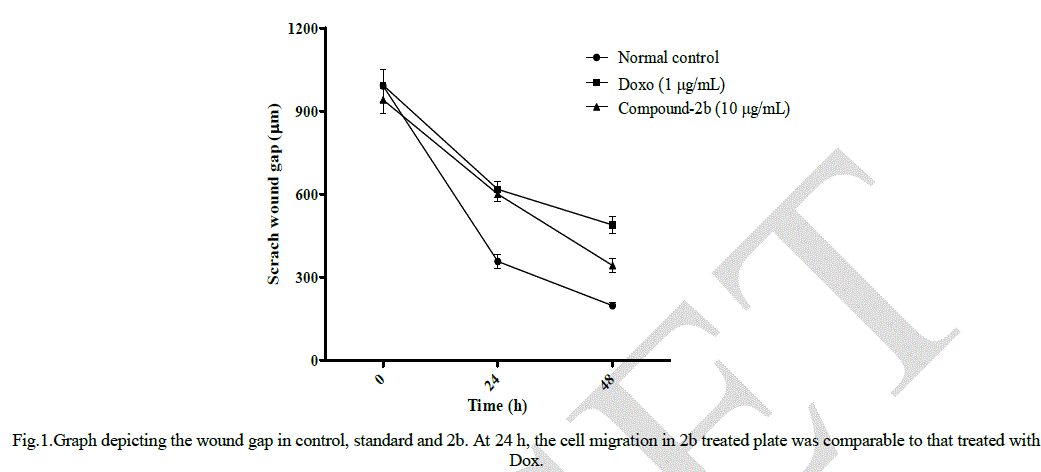 |
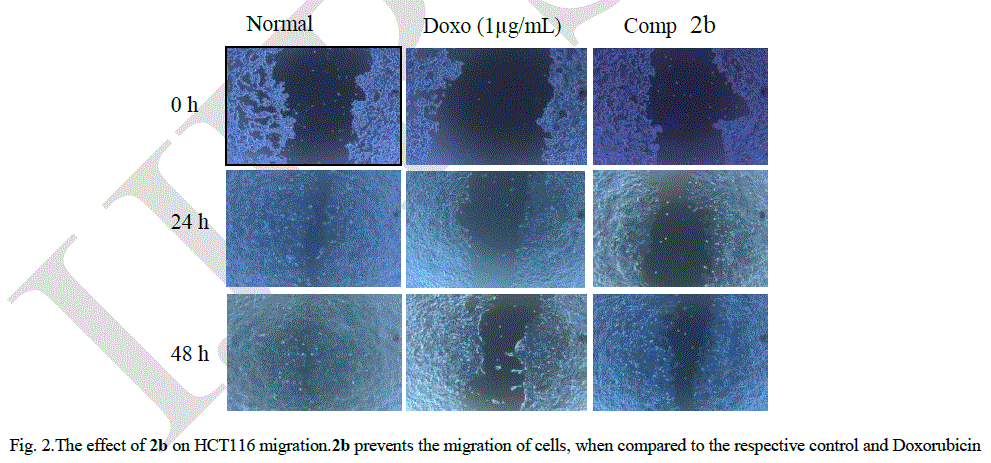
| Fig 2 shows cell migration across a wound inflicted in an HCT116 cell monolayer for the control, standard doxorubicin (1 μg/mL) and 2b (10 μg/mL). At the end of 24 h, the wound gap in cells treated with 2b was almost same as that in the case of standard, showing the efficacy of 2b in restricting cell migration. Fig 2 clearly indicates that after 48 h following application of the wound, the HCT116 cells were less motile and could not close the wound gap (p < 0.05), whereas in the control, the cells could completely heal the wound. |
 |
| EMT plays important physiological role during wound healing and is also crucial for the acquisition of tumoral invasiveness, the initial step of metastatic cascade in cancer [29].The best docking pose where 2b lies deep into the Snail 1 binding cavity representing the ligand-protein interaction and the binding mode is depicted in Fig.3. 2b have hydrogen bonded interaction with the surrounding cage of amino acids within the binding pocket of Snail 1 (Fig. 4). |
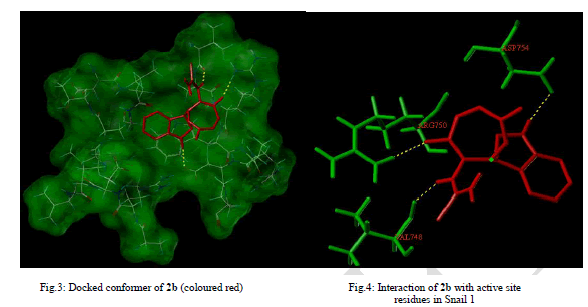 |
| Docking of 2b resulted in high scoring orientations and favourable docking score of 4.06 presumably due to three Hbonds and also hydrophobic interactions with the receptor molecule. The oxygen at the fifth position of oxazepine ring was H-bonded to Arg 750, hydrogen attached to nitrogen of indole ring to Asp 754 and hydrogen attached to N of the side chain to Val 748. High molecular weight (>500 Dalton) and high numbers of Hydrogen bond acceptors (>10) and donors (>5) may impair permeability across membrane bilayer. The Lipinski parameters for 2b (mol. wt - 316, LogP - 1.2099, H-bond acceptors - 4 and H-bond donors - 3) suggests that it may have better oral bioavailability. Polar surface area of 128 Å2 indicated the better oral absorption, intestinal permeability and passive transport of 2b. |
IV. CONCLUSION |
| Four new oxazepines were synthesized by the cycloaddition reaction between Schiff bases and maleic anhydride. 1-[2- (2,3-dihydro-1H-indol-3-yl)-4,7-dioxo-4,7-dihydro-1,3-oxazepin-3(2H)-yl]thiourea displayed cytotoxic and antimigratory properties. The docking studies revealed the compound as an excellent snail 1 inhibitor. Hence there is ample scope in taking up this oxazepine for further studies. |
| Experimental |
| 1-[2-(4-hydroxyphenyl)-4,7-dioxo-4,7-dihydro-1,3-oxazepin-3(2H)-yl]thiourea(2a) Yellow solid (80%) m.p.200-202 oC; IR (KBr) [cm-1]: 3545 (OH str.), 3471, 3363 (NH2 str.), 3193 (NH str.), 3050 (Ar. C-H str.), 1689 (C=O str.), 1550 (Ar. C=C str.), 1319 (C-O str.), 1234 (C=S str.); 1H NMR DMSO d6, 400MHz, 10.5 (1H, OH), 9.8 (1H, NH), 7.32-8.1 (4H, Ar. H), 7.24 (1H, O-CH-N), 6.34-7.01 (2H, CH=CH), 2.5(2H, NH2); MS (m/z): 293 (M+).Anal.calcd. For C12H11N3O4S; C, 49.14; H, 3.75; N, 14.33. Found: C, 49.20; H, 3.76; N, 14.35. |
| 1-[2-(2,3-dihydro-1H-indol-3-yl)-4,7-dioxo-4,7-dihydro-1,3-oxazepin-3(2H)-yl]thiourea(2b) Brown solid (86%) m.p.270-274 oC; IR (KBr) [cm-1]: 3448, 3317 (NH2 str.), 3180 (NH str.), 3085 (Ar. C-H str.), 1689 (C=O str.), 1542 (Ar. C=C str.), 1315 (C-O str.), 1226 (C=S str.); 1H NMR DMSO d6, 400MHz, 10.1 (1H, indole NH), 9.8 (1H, NH), 7.33-8.2 (5H, Ar. H), 7.22 (1H, O-CH-N), 6.27-6.94 (2H, CH=CH), 2.5(2H, NH2); MS (m/z): 316 (M+).Anal.calcd. For C14H12N4O3S; C, 53.16; H, 3.8; N, 17.72. Found: C, 53.23; H, 3.9; N, 17.76. |
| 1-[2-(2,3-dimethoxyphenyl)-4,7-dioxo-4,7-dihydro-1,3-oxazepin-3(2H)-yl]thiourea(2c) Yellow solid (80%) m.p.198-200 oC; IR (KBr) [cm-1]: 3436, 3255 (NH2 str.), 3155 (NH str.), 3047 (Ar. C-H str.), 2947 (CH3 asym str.), 2831 (CH3 sym str.), 1728 (C=O str.), 1535 (Ar. C=C str.), 1320 (C-O str.), 1272 (C=S str.); 1H NMR DMSO d6, 400MHz, 9.8 (1H, NH), 7.43-8.2(3H, Ar. H), 7.26 (1H, O-CH-N), 6.361-7.081 (2H, CH=CH), 3.89 (3H, OCH3), 3.81 (3H, OCH3), 2.5 (2H, NH2); MS (m/z): 337 (M+); Anal. calcd. For C14H15N3O5S; C, 49.85; H, 4.45; N, 12.46. Found: C, 49.94; H, 4.47; N, 12.49. |
| 1-[2-(4-hydroxy-3-methoxyphenyl)-4,7-dioxo-4,7-dihydro-1,3-oxazepin-3(2H)-yl]thiourea(2d) Yellow solid (85%) m.p.144 oC; IR (KBr) [cm-1]: 3523 (OH str.), 3433, 3278 (NH2 str.), 3155 (NH str.), 3033 (Ar. C-H str.), 2939 (CH3 asym str.), 2830 (CH3 sym str.), 1720 (C=O str.), 1512 (Ar. C=C str.), 1320 (C-O str.), 1280 (C=S str.); 1H NMR DMSO d6, 400MHz, 10.8 (1H, OH), 9.78 (1H, NH), 7.39-8.1 (3H, Ar. H), 7.27 (1H, O-CH-N), 6.33-6.94 (2H, CH=CH), 3.88 (3H, OCH3), 2.5 (2H, NH2); MS (m/z): 323 (M+); Anal. calcd. For C13H13N3O5S; C, 48.29; H, 4.02; N, 13.00. Found: C, 48.35; H, 4.04; N, 13.04. |
References |
|