ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Suneetha1 T.B, Dr. S M Gopinath1, Divya T.K2, Amarshankar2, Narasimha Murthy.T.P3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The acquired immunodeficiency syndrome (AIDS) is a result of human immunodeficiency virus (HIV) infection which leads to severe suppression of immune functions. Numerous plant derived compounds have been found to inhibit different steps in the HIV replication cycle. Sonchus oleraceus is belongs to Asteraceae family have some medicinal property. The plant is a rich source of many biotic compounds which are of medicinal importance. The study was undertaken to investigate the anti-HIV and anti-bacterial activities of coumarin compounds extracted from the Sonchus oleraceus. Microwave assisted extraction method is followed for the extraction process using three solvents i.e. ethanol, methanol and water at different temperature for 5 min followed by one or two cycle extraction method. The obtained extracts were subjected to identification of coumarin using three different methods via, FeCl3, fluorescence method and other coumarin test. Extracts were also subjected to phytochemical analysis to check the presence of other phytochemical compounds. Screening of coumarin compound for the inhibitory action against protease enzymes involve in the replication of HIV.The methanol extracts of Sonchus oleraceushave shown significant inhibition of protease activity as compared to standard (lopinavir).
Keywords |
| HIV, AIDS, Sonchus oleraceus, Protease enzyme, lopinavir, coumarin |
INTRODUCTION |
| AIDS is a pandemic immunosuppressive disease which results in life-threatening opportunistic infections and malignancies. Since a retrovirus, designated human immunodeficiency virus (HIV) has been clearly identified as the primary cause of this disease1-2. The replicative cycle of HIV comprises ten steps that could be considered suitable targets for chemotherapeutic intervention3. A number of laboratories are actively involved in the development of antiviral agents that interfere with HIV at different stages of viral replication4-5. The high mutation rate of HIV frequently results in the rapid development of resistance towards the drugs used, and an attempt has been made to circumvent this problem by using a combination of drugs6. A phytotherapeutic approach to modern drug development can provide many invaluable drugs from traditional medicinal plants. Over the last decade, antiviral researchers have also turned into many traditional folk medicines, invariably a “cocktail” of natural products, to uncover the scientific basis of their remedial effects. Many plant products are being used by patients with AIDS in some countries without any scientific proof that they possess anti-HIV activity. Traditional healers are now offering their remedies for scientific evaluation, and a number of studies provide information on the inhibitory activity against HIV of selected plants7-9. A large number of plant-derived substances have been described that exhibit anti-HIV activity, e.g., alkaloids, polysaccharides, lignans, flavonoids, coumarins and terpenes10-12. |
MATERIALS AND METHODS |
| The plant was collected locally from Tumkur district, India in April 2011. The shade dried plant was ground into fine powder and the total mass was subjected to extraction by microwave assisted extraction method13 with water, Ethanol and Methanol in a closed-vessel system (GMS 17M 07 WHGX SOLO Microwave).Two grams of powdered sample were subjected to extraction in a vessel with of solvent up to the volume of 2ml. Microwave assisted extraction method is followed for the extraction process using three solvents at different temperature for 5 min followed by one or two cycle extraction method at different temperature. The resulting extracts were kept in refrigeration for further studies. |
| Phytochemical analysis: |
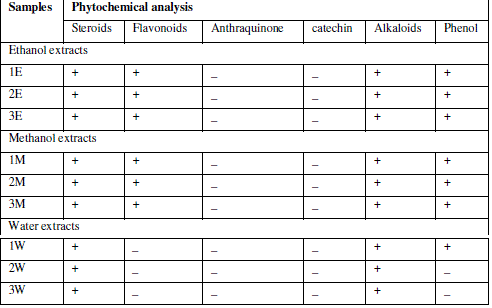
| Phytochemical analysis for major phytoconstituents of the plant extract was undertaken using standard qualitative methods as described by various authors. Phytochemical analysis was carried out for saponins, flavonoids, terpenoids, steroids, phenol, alkaloids,anthraquinone, Quinone, Catechin14, tannins15. |
| A) Steroids and Terpenoids |
| 10mg of the extract was dissolved in chloroform. Few drops of acetic anhydride were added followed by 1 ml of con Sulphuric acid. The blue colouron chloroform layer which changes to green shows the presence of steroids, whereas the appearance of pink colour in chloroform layer shows the presence of terpenoids. |
| B) Alkaloids |
| 10mg of the extract is dissolved in con HCL and filtered. A few drops of solution are poured into the center of watch glass. Mayer reagent is added on the sides of the watch glass with the help of a glass rod. Formation of a gelatinous white precipitate at the junction of the two liquid shows the presence of alkaloids. |
| C) Flavonoids |
| 10mg of the extract was dissolved in methanol. Magnesium turnings were added into this followed by con HCL. A magenta colour shows the presence of Flavonoids. |
| D) Saponins |
| The extract was dissolved in water and shaken well. Froth which last for a long time shows the presence of saponins |
| E) Tannins |
| 10 mg of the extract was boiled with 1 ml water for 30 min. The extract is filtered clear and to this 0.5 ml 2% gelatin was added. A curdy white precipitate indicates the presence of tannin. |
| F) Phenolic compounds |
| The extract was dissolved in alcohol and 1 drop of neutral ferric chloride was added to this. The intense colour indicates the presence of phenolic compound. |
| H) Anthraquinone |
| To the extract Magnesium Acetate solution was added the pink colour developed indicates the presence of Anthraquinone. |
| I) Quinone |
| Few mg of the substrate in alcohol is treated with sulphuric acid. The colour developed indicates the presence of Quinone. |
| J) Catechin |
| Few mg of the substrate in alcohol is treated with a few drops of Ehrlish reagent and a few drops of concentrated HCl. The pink colour developed indicates the presence of catechin. |
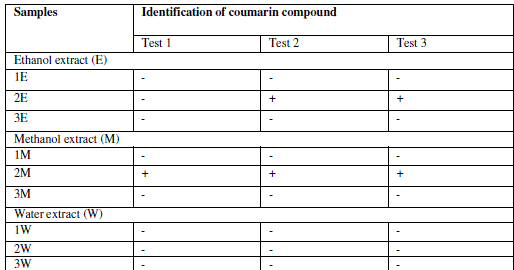
| Identification of coumarin in extracts |
| Test 1: |
| With the concentrated alcoholic extract of drugs few drops of alcohol ferric chloride (FeCl3) solution were added. The formation of deep green color, which turned yellow on the addition of conc. Nitric acid (HNO3), indicates the presence of coumarins. |
| Test 2: |
| The alcoholic extract of the drug was mixed with 1N sodium hydroxide (NaOH) solution (one mile each). Development of blue-green fluorescence indicates presence of coumarins. |
| Test 3: |
| 3ml of methanol extract was evaporated to dryness in a vessel and the residue was dissolved in hot distilled water. It was then cooled and divided into two test portions, one was referenced, second was the test. In the second test tube, 0.5ml of 10% ammonium hydroxide (NH4OH) was added. The occurrence of intense/fluorescence under UV light is a positive test for the presence of coumarins and derivatives. The experiment was carried out for all the experiment in three replicates 16,17. Enzyme inhibition assay |
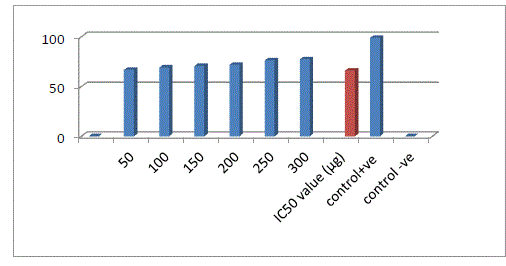
| Protease has a quite resemblance in proteolytic activity with HIV-protease one key enzyme of HIV-1 life cycle as both of them belongs to same aspartateenzyme family18. This enzyme was used as a substitute of HIV-1 protease to check out anti- HIV activity of plant extracts in the present investigation.To measure the inhibition of the protease enzyme (Sigma Aldrich) used with their corresponding substrate (Sigma Aldrich). For the assay, 50 μg protease, 800 μg substrate and different concentration of the purified coumarin sample was taken in 500 μl of reaction mixture. The mixture was allowed to incubate at 370C, after 20 min, 700 μl of 5% TCA was added to stop the reaction. It was then centrifuged at 14, 000 g for 5 min and the supernatant was collected. Optical Density (OD) was recorded spectrophotometrically at 280 nm. Separate blanks were used or both positive and negative controls as well as for sample. For positive control enzyme and substrate were taken and followed the above procedure and for negative control protease was taken as a well-known inhibitor of HIV-protease, lopinavir was taken. Each sample was taken in triplicate, so this assay gives reproducible results. Percentage of inhibition was calculated by using a formula. Inhibition (%) = [(OD of negative control - OD of sample) /OD of negative control] ×100 |
| Antibiotic Assay |
| The test organisms used in the present study included Klebselaspp.,staphylococcus aureus, Proteus mercilessand Serratia spp.For screening of antibacterial activity of different plant extract agar well diffusion method19-20 was employed. The test bacterial cultures were prepared as follows: a loop full of cells from master culture was inoculated into 100 ml sterilized nutrient broth contained in 250 ml conical flack and inculcated on an orbital shaker at 37°C for 12hr. The cultures obtained were centrifuged in a cooling centrifuge at 10,000 rpm for 15 min. The resulting pellet was resuspended in sterilized cool distilled water for washing and repeated the centrifugation twice. After the final washing the cells were diluted with saline solution (1:1000) to give suspensions of about 1×108 microorganisms per ml21. About 0.1 ml of the suspension was then spread on sterilized nutrient agar test plates and thereafter, the wells were cut out using sterile cork borer. Then, about50μl of each extract were poured into these wells. The plates were then incubated at 370C for 24 hrs. The antimicrobial activity was evaluated by measuring the diameter of inhibition zones were calculated. |
RESULTS |
 |
 |
 |
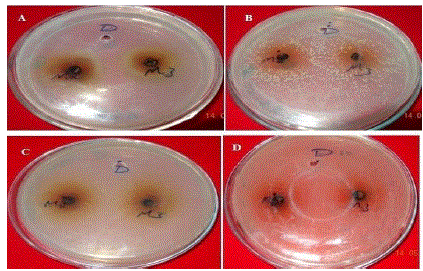 |
| Anti-bacterial property of M2 and M3 test samples. A- Antibacterial property of test samples against the Klebsela spp., B- antibacterial property of test samples against the staphylococcus aureus, C- antibacterial property of test samples against the Proteus merciless, D- antibacterial property of test sample against the Serretia. M-Methanol extracts, D- DMSO, 2: Two cycles at 500 C, 3: one cycle at 700 C |
DISCUSSION |
| Extraction was performed by the microwave assisted extraction method using three solvents (ethanol, methanol, water) followed by one or two cycle extraction at different temperatures.Table 1 shows the phytochemical analysis of the methanolic, ethanolic and aqueous extracts. It was performed in order to know the presence of different phytocompounds such as alkaloids, flavonoids, saponins, tannins, terpenoids, phenol, quinine etc. Results obtained showed the presence of steroid and alkaloids in all six extracts and flavonoids in methanolic and ethanolic extracts and it is absent in aqueous extracts. Anthraquinone and catechin were not found in all six extracts. Phenolic compound was present in the ethanolic and methanolic extracts and it was absent in aqueous extracts.Table 2 shows the presence of the coumarin in methanolic extracts in all three tests. Presence of coumarin is confirmed in two cycled methanolic extracts.Table 3 indicates the anti HIV property of a coumarin compound purified from the methanolic extracts of Sonchus oleraceus. Coumarins from the M2 extracts have shown anti HIV property.Anti HIV activity was evaluated by using protease enzyme. M2 extracts have shown inhibitory activity with 66.114 IC50 value. Figure1 shows percentage of inhibition was increased with increasing the concentration of the sample.From these results we confirmed the anti HIV property of coumarin compound and it acts as an inhibitor for the protease enzyme, further studies are needed to know the mechanism of coumarins on the protease enzyme as an inhibitor.Figure 2 indicates the antibacterial activity of coumarin extracts was tested against the four pathogenic bacteria. The activity was maximal in the coumarins from the M2 extracts. Purified coumarins of M2 extracts exhibited greater antibacterial potential against the four pathogenic bacteria. Table 4 shows the zone of inhibition against the four pathogenic bacteria for methanolic extracts. Coumarins extracts have shown more antibacterial potency against Klebsela spp. With 28mm and 27 mm inhibition zone for M2 and M3 extracts respectively. This extracthas also shown 22mm and 21mm of inhibition zone against staphylococcus aureus, 16mm and 15mm of inhibition zone against Proteus merciless and 12mm and 11mm of inhibition zone against Serretia for M2 and M3 extracts respectively.From these results we confirmed antibacterial activity of coumarin compounds against four bacterial species and also come-on extracts have shown more antibacterial potency against Klebsela spp. |
References |
|