ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Ganesh Mhaske, PankajNilkanth, AvinashAuti, Sachin Davange and SharadShelke* Research Center, Department of Chemistry, S.S.G.M. College, Kopargaon, Dist-Ahmednagar (MS) 423601, India. |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In this study the investigations on the synthesis of Schiff bases derived from 3-formyl chromones and various aromatic anilines are reported. The reactions are carried out under MW (microwave) irradiation using aqueous media. This afforded the formation of product(s) in excellent yield (81.17 to 88.14 %). Further, all the synthesized compounds were screened for their in vitro antibacterial and antifungal activity against various strains by using the agar diffusion method. The Schiff bases were found to exhibit good to excellent activity against bacterial as well as fungal species.
Keywords |
| Aqueous media, MW, 3-formylchromone, bioactivity, antibacterial, antifungal |
INTRODUCTION |
| Schiff bases are biologically1 as well as synthetically2 important nitrogen containing compounds having azomethine group (-CH=N-) and are formed by condensation between primary amines and carbonyl compounds.Schiff bases have been associated with various significant catalytic3 and photochromic properties.4Those are also known to show the additional abilities5 to the metal ions to form stable complexes. It is well known from the literature that Schiff bases exhibit an antibacterial6 and antifungal7 bioactivity. Isoniazid Schiff bases have been studied for their anti-tubercular activity.8Various researchers have paid attention towards synthesis9 and their biological evaluation.10, 11 Schiff bases also have played a key role in the synthesis of metal complexes.12 At this point of view, in the last couple of years researchers report improvements in the synthesis procedures of metal complexes13 of Schiff bases and their pharmacological studies14. In the field of coordination chemistry Schiff bases are used as chelating agent.15, 16 Some metal complexes of Schiff bases have studied for their synthetic oxygen carriers in the biological system.17 Due to biological significance connected with Schiff bases, several research groups have increased their interest in the development of newer synthetic methods.18 The researcher has true load to investigate the environmentally benign methods and reagents to prepare the chemicals because conventional methods have several drawbacks and this is overcome in microwave assisted synthetic method. 19 Developments of green methods need to reduce the use of solvent in the reaction because it generates waste. 20 From a strict green chemistry point of view, the real solution to this problem would be to carry the reactions without any solvent. However, carrying a reaction in a solvent is very essential to facilitate mass and heat transfer. Considering this it is a major challenge to use environmentally safe solvent such as water or use solvent-free synthetic methods. 21 Recently, various reactions have been reported in the literature for the use of water as a solvent.22-25 Various organic reactions were carried in water because it is cheap, safe and clean solvent.26 Due to these advantages are connected with water, the industry prefers to use water as a solvent rather than toxic organic solvents.27 Microwave assisted organic reaction methods are superior to conventional method because it occur more rapidly, safely and with the highest chemicals yields.28, 29 Last few decades, increasing the number of publications in microwave-assisted synthesis includes almost all types of reactions.30-32 In a continuation of our efforts in the field of green synthesis33,34 and versatile applications associated with Schiff bases, herein we report the synthesis and biological assessment of Schiff bases. |
II. RESULT AND DISCUSSION |
| Chemistry |
| The substituted 3-formylchromones 1 were synthesized by the Vilsmeier-Hack method.35In present investigation various substituted 3-formylchromones 1 are treated with substituted anilines 2 in aqueous media with microwave as an energy source to yield the Schiff bases 3 (Scheme I). The progress of the reaction and the purity of the compounds were performed by TLC (thin-layer chromatography). The resulting product was recrystallized from ethanol. Considering the difficulties in achieving high purity of compounds 3 by recrystallization technique, we purified the compound 3 by preparative TLC. |
| SCHEME-I |
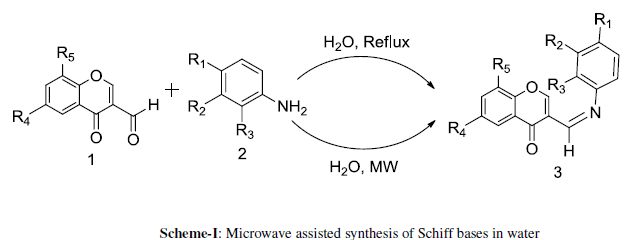 |
| Initially, as a benchmark the reactions were performed under conventional refluxing method and low yields were observed (Table 1). On the other hand the same reactions when performed using microwave techniques were very clean and required less time for completion with appreciably higher yields. In both the methods water was used as solvent for energy transfer media. |
| Optimization studies |
| We have been using different solvents, such as ethanol, methanol, toluene, dioxane and tetrahydrofuran (THF), under microwave irradiation at 150oC and 210 w for 2.6-15 min to obtain 3a in 45.11–84.15% yield. (Table 1). It can be observed that the reaction using water as the solvent resulted in higher yields and shorter reaction times than those using other solvents. |
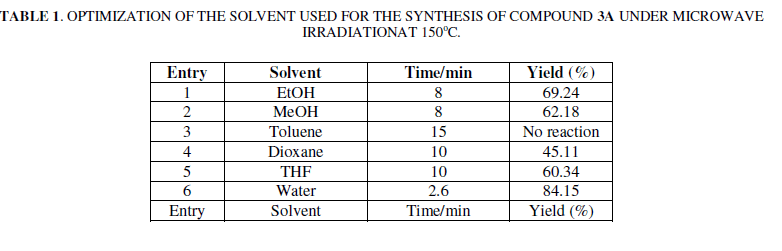 |
| Using water as a solvent, the reactions of variously substituted 3-formylchromones 1 and substituted anilines 2 were carried out to get substituted Schiff bases 3a-3g in 81.17 to 88.14 % yields (SCHEME-I, TABLE 2). January |
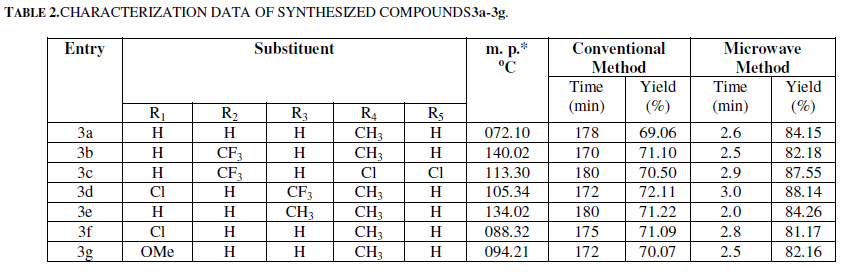 |
| *The Melting Point was taken in M. P. apparatus Model: KI-11 (MP-D), Make: Kumar Sales Corporation, Mumbai (India). |
| As observed from Table 2, the compounds 3 are obtained in good yield within 2-3 minutes when reactions were conducted using microwave irradiation. Each experiment was repeated three times to confirm the consistency of the results. Compared to microwave assisted reactions the non-conventional method (refluxing) required 170-180 minutes for the completion of the reaction and yields are found to be comparatively low. So, we report here the green methodology for the synthesis of Schiff bases in a very friendly solvent–water and microwave as an energy source. The structures of the tilted compounds were confirmed by using spectroscopic techniques which include IR (infrared), 1H-NMR (proton nuclear Magnetic resonance), 13C-NMR (carbon thirteen nuclear Magnetic resonance) and MS (mass) spectroscopy. |
| Antimicrobial activity: |
| The newly synthesized compounds were tested for antimicrobial activity in order to determine their potential application in microbial chemotherapy. The antimicrobial data are represented in TABLE 3. |
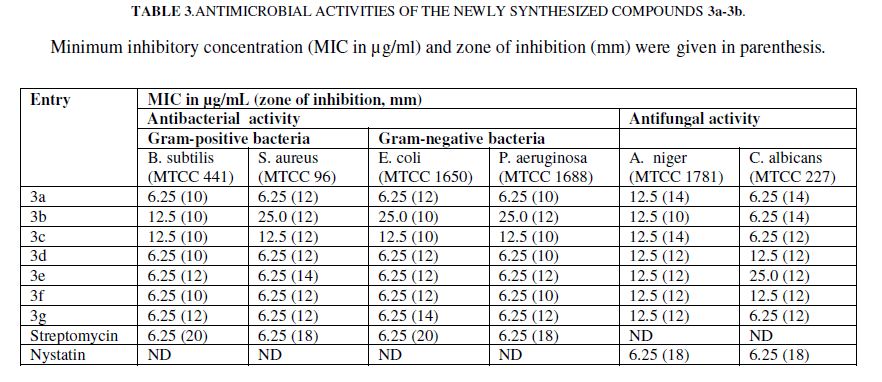 |
| Note: ND- Not determined, MIC values were evaluated on a concentration range between 6.25 and 200 μg/ml. |
| Structure activity relationship |
| Antibacterial activities |
| Schiff bases 3a, 3d, 3e, 3f and 3g exhibited higher antimicrobial activity against all the bacterial strains viz. B. subtilis (MTCC 441), S. aureus (MTCC 96), E. coli (MTCC 1650) and P. aeruginosa (MTCC 1688). The MIC values (6.25 ïÂÂg/mL) and the zones of inhibition (~ 12 mm) of Schiff bases 3a, 3d, 3e, 3f and 3g were comparable with the reference compound, Streptomycin. The observed effects may be attributed to the methyl group at R4 in these Schiff bases. While less inhibitory activity was shown by Schiff base 3c against all four bacterial strains where methyl group was replaced with Cl. Schiff bases having R1 as OMe and R4 as methyl group i.e. 3g exhibited a MIC of 6.25 ïÂÂg/mL, whereas Schiff bases having R2 as CF3 group in 3b and 3c exhibited a MIC of 12.5 ïÂÂg/mL, suggesting that the activity increases with the presence of methyl groups at R4. |
| Antifungal activities |
| Similar to antibacterial activity, the Schiff bases 3a, 3b, 3c and 3g exhibited excellent antifungal activity compared to reference compound Nystatin against C. albicans. Nearly all Schiff bases 3a-3g showed comparatively higher MIC values against A. niger, while 3d and 3f showed higher MIC values against C. albicans with reference compounds Nystatin. Antifungal activity of Schiff bases having R4 as methyl group 3a and 3b showed a MIC of 6.25 ïÂÂg/mL, whereas when R1 and R3 as methyl group 3e showed a MIC of 6.25 ïÂÂg/mL. Schiff bases having R1 as OMe and R4 as methyl group 3g exhibited a MIC of 25.0 ïÂÂg/mL. Schiff bases having R1 as Cl and R4 as methyl group 3d and 3f showed a MIC of 12.5 ïÂÂg/mL, it means that activity relationship is not specific for specific substituent. |
III.EXPERIMENTAL |
| All the recorded melting points were determined on M. P. apparatus Model: KI-11 (MP-D), Make: Kumar Sales Corporation, Mumbai (India) and are uncorrected. All experiments under microwave irradiation were carried out in microwave synthesis system 700W model manufactured by RAGA’s Scientific Microwave Synthesis System Pvt.Ltd, Pune, India has a maximum power output of 700W and 2450 MHz frequency. FTIR spectra were recorded on a Perkin- Elmer FTIR spectrophotometer in KBr pellets. The NMR spectra were recorded on a Bruker 400 MHz instrument using DMSO-d6 (deutrated dimethyl sulfoxide) as a solvent and TMS (tetramethylsilane) as an internal standard; the chemical shifts (ï¤) are reported in ppm (part(s) per million) and coupling constants (J) are given in Hertz. Signal multiplicities are represented by s (singlet), d (doublet), t (triplet), dd (double doublet), and m (multiplet). Mass spectra were recorded on a Finnigan mass spectrometer. TLC was performed on pre-coated silica gel glass plates (Kieselgel 60, 254, E. Merck, Germany) |
| General method for the synthesis of (6-methyl-3-((phenylimino)methyl)-4H-chromen-4-one (3a) By conventional method |
| 6-Methyl-4-oxo-4H-chromene-3-carbaldehyde 1 (188 mg, 0.001 mol) and aniline 2 (93 mg, 0.001 mol) were taken in 100 mL RBF with water (5 mL). The reaction mixture was heated under reflux for 178 min. Progress of the reaction was monitored with the help of TLC (10 % Ethyl acetate/n-hexane). After completion of heating, the reaction mixture was cooled to RT and the product obtained was separated by filtration. Purification method was applied for recrystallization. The pure compound 3a (181.62 mg, 69.06 %) was isolated by preparative TLC. The formation of compounds 3a was confirmed by m.p, mixed m.p. and spectral studies. This typical experimental procedure was followed to prepare other analogues of this series. The compounds synthesized by above procedures have been listed in Table 2 with their characterization data. |
| By microwave method |
| A 50 mL borosilicate glass beaker was charged with 6-methyl-4-oxo-4H-chromene-3-carbaldehyde 1 (188 mg, 0.001 mol) and aniline 2 (93 mg, 0.001 mol) in water (5 mL). The reaction mixture was irradiated inside a microwave oven for 2 to 3 min at an output of 300 watts power, with short interruptions of 15 second. Progress of the reaction was monitored by TLC (10 % Ethyl acetate/n-hexane). After completion of the reaction, the product obtained was separated by filtration. The product was purified by recrystallization from ethanol. The pure compound 3a (195.01 mg, 84.15 %) was isolated by preparative TLC. The formation of compounds 3a was confirmed by m.p, mixed m.p. and spectral studies. This typical experimental procedure was followed to prepare other analogues of this series. The compounds synthesized by above procedures are listed in Table 2 with their characterization data. |
| 3a: IR (KBr) v/cm-1: 3062, 2965, 2936, 1668 (–C=O), 1541 (N=C),1458, 1364, 1328, 1271, 1056, 1039, 928, 767.1HNMR (400 MHz, DMSO-d6): 2.50 (3H, s, CH3), 6.79-8.35 (9H, m, HAr), 10.23 (1H, s, Chromone =CH). 13C-NMR (400 MHz, DMSO-d6): 19.19 (CH3), 101.45, 105.4, 108.8, 112.9, 116.2, 119.11, 122.12, 124.19, 128.1, 131.6, 133.16, 141.12, 141.12, 144.19, 153.12, 182.8 (C=O); MS (m/z): 264 (M++1). |
| 3b: IR (KBr) v/cm-1: 3064, 2975, 2925, 1655 (–C=O), 1561 (N=C),1448, 1374, 1330, 1281, 1118 (-C-F), 1068, 1040, 925, 788. 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 2.51 (s, 3H, CH3), 6.70-8.21 (m, 8H, HAr), 9.88 (s, 1H, CH). 13CNMR (400 MHz, DMSO-d6, δ, ppm): 20.16 (CH3), 83.01 (CF3), 104.47, 112.3, 113.7, 117.1, 118.03, 120.10, 125.09, 129.2, 130.2, 134.9, 140.6, 141.74, 143.3, 146.2, 154.3, 183 (C=O); MS (m/z): 332 (M++1). |
| 3c: IR (KBr) v/cm-1: 3080, 2975, 2921, 1652 (–C=O), 1558 (N=C), 1464, 1334, 1308, 1284, 1167, 1120 (-C-F), 979, 791. 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 6.08-7.79 (m, 7H, Ar), 10.07 (s, 1H, CH). 13C-NMR (400 MHz, DMSOd6 , δ, ppm): 83.59 (CF3), 100.64, 103.34, 111.23, 119.91, 120.98, 122.40, 123.41, 124.25, 125.11, 130.10, 130.63, 133.17, 140.25, 145.88, 150.02, 177.8 (C=O); MS (m/z): 386 (M++1, 100%), 387 (M++2, 66.0 %), 389 (M++3, 10.2 %). |
| 3d: IR (KBr) v/cm-1: 3088, 2976, 2920, 1655 (–C=O), 1575 (N=C), 1449, 1388, 1318, 1277, 1123 (-C-F), 1061, 1038, 979, 795, 814. 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 2.49 (s, 3H), 6.65-8.52 (m, 9H, HAr), 10.37 (s, 1H, CH). 13CNMR (400 MHz, DMSO-d6, δ, ppm): 20.45 (CH3), 83.81 (CF3), 106.52, 117.69, 118.2, 120.1, 122.1, 125.4, 126.4, 128.6, 132.7, 135.9, 136.8, 142.2, 143.0, 155.2, 160.5, 182.40 (C=O); MS (m/z): 366 (M++1). |
| 3e: IR (KBr) v/cm-1: 2923, 2853, 1644 (–C=O), 1590 (N=C), 1448, 1368, 1307, 1278, 1023, 948, 746. 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 2.51 (s, 3H, CH3), 2.52 (s, 3H, CH3), 6.18-8.28 (m, 8H, HAr), 10.11 (s, 1H, CH). 13C-NMR (400 MHz, DMSO-d6, δ, ppm): 18.44 (CH3), 18.72 (CH3), 84.09 (CF3), 101.64, 105.27, 113.23, 119.91, 124.65, 124.77, 125.92, 130.44, 130.74, 141.25, 145.98, 148.85, 152.02, 153.64, 181.79 (C=O); MS (m/z): 278 (M++1). |
| Minimum inhibitory concentration (MIC) measurement |
| The antibacterial activities of the synthesized compounds 3a-3g were determined by agar diffusion methods. The bacterial strains viz. Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 96), Pseudomonas aeruginosa (MTCC 1688), and Escherichia coli (MTCC 1650) strains were selected for the study. Briefly, 0.1 mL of overnight grown respective bacterial culture was spread over the nutrient agar plates. The wells of 6 mm diameter were prepared on the nutrient agar plates and filled with diluted test compounds separately. For comparison, DMSO (dimethyl sulfoxide) and antibiotic Streptomycin were used as a solvent control and as a referral antibacterial agent, respectively. Inoculated plates were then incubated at 37°C for 24 h and the resulting zones of inhibition (in mm) were measured. The minimum inhibitory concentrations at which no observed growth was taken as the MIC value. |
| The compounds were screened for their antifungal activity on the fungal strains Aspergillusniger (MTCC 1781) and Candida albicans (MTCC 227). Fungal suspension (0.1 mL) was spread on Potato dextrose agar plates. The wells of 6 mm diameter were prepared on the inoculated plates and filled with diluted test compounds separately. For comparison, DMSO and antibiotic Nystatin were used as solvent control and reference antifungal agent, respectively. Immunized plates were then incubated at 30°C for 2-3 days and the resulting zones of inhibition (in mm) were measured. The minimum inhibitory concentrations at which no fungal growth observed was recorded as the MIC value. |
IV. CONCLUSION |
| In conclusion, various Schiff bases 3 derived from 3-formyl chromone1 and aromatic amines 2 were synthesized in aqueous media using conventional and microwave technique. It was found that microwave technique is superior green alternative method compared to the conventional synthesis of titled compound 3 for its simple operation, lower temperatures, shorter reaction times, and higher yields. These reactions were successfully carried in aqueous medium and provides a highly atom efficient green pathway for the synthesis of titled compound. It was concluded that Schiff bases showed good to excellent antimicrobial activities against various bacterial and fungal strains. Among them 3e, 3g and 3a, 3b exhibited same MIC values when compared with reference compound Streptomycin and Nystatin, respectively. |
ACKNOWLEDGMENT |
| The author (SNS) is thankful to the UGC (WRO) for funding. We are also grateful to the Principal Dr. K. H. Shinde and Dr. A. B. Nikumbh, (HOD), S.S.G.M. College, Kopargaon, Ahmednagar (MH) for providing research facilities and constant encouragement. |
References |
|