ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Mahanti1, Rishi Sharma2, Ashwini Kr. Singh3, Neelima Sharma4, S. K. Pradhan5, P. K. Barhai6
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Diamond like carbon (DLC) films are deposited on glass substrates by using radio frequency plasma enhanced chemical vapour deposition technique with the variation of RF power. DLC films are deposited by using argon and acetylene plasma, at pressure 5×10-2 mbar for 60 minutes. Various properties like surface morphology, hydrophobicity, nanohardness, and structural properties are studied by using atomic force microscope, contact angle measurement setup, nano-hardness tester and Raman spectrometer, respectively. Systematic variation in surface morphology with RF power has been observed. It has been observed that the morphology of the films is correlated with the hardness, sp3 content, roughness, and contact angle. Observed results have been explained using Raman spectra.
KEYWORDS |
| DLC, AFM, Surface morphology, nano-Hardness, Raman spectra, Contact angle. |
I. INTRODUCTION |
| Diamond like carbon (DLC) films are amorphous in structure, these films contain both sp2 (graphite) and sp3 (diamond) bonded carbon. These films have attractive properties like high electric resistivity, chemical inertness, high thermal conductivity, high hardness, biocompatibility etc. [1-5]. These properties make DLC films a suitable candidate for various applications like hard coatings for cutting tools, wear resistance coatings, low friction coatings, biomedical coatings etc. [3-7]. As opposed to diamond which occurs naturally and can be synthesized at both high and low pressure, DLC is not a natural material and can only be produced at low pressure. DLC is not a single material, but a broad class of amorphous carbon based materials, consists not only of the amorphous carbon but also of hydrogenated alloys [3]. DLC films have two disadvantages: (i) low deposition rate and (ii) high internal stress [8-10]. Unlike diamond, it cannot be obtained as thick monolithic shapes, at least with the present technology. However, DLC has properties similar to CVD diamond, it is easier to process without the high-temperature substrate requirements and with little restriction on size [11]. |
| DLC has good mechanical properties similar to diamond, but these are achieved in an isotropic disordered manner with no grain boundaries. The sp3 bonding of DLC confers many of the beneficial properties of diamond itself such as its hardness, chemical and electrochemical inertness and wide band gap. Formation of various surface structures and its correlation with sp2/sp3 bonded carbon has not been studied extensively. In the present investigation, the effect of sp2/sp3 bonded carbon on surface morphology has been reported. |
II. RELATED WORK |
| The first DLC film was prepared by Aisenberg and Chabot in 1971 using ion beam deposition [12]. Different deposition techniques like ion assisted sputtering, cathodic/ anodic vacuum arc, magnetron sputtering, DC/ RF/ microwave plasma enhanced chemical vapor deposition (PECVD) techniques, pulse laser deposition are commonly used for the deposition of DLC films [4, 13-19]. The characteristic features of most of the processes for growing dense carbon films are the energetic species usually in the range of 50 to 500 eV that hit the surface of the growing films [3]. DLC has high hardness, spanning the range from 10 to 30 GPa, associated with high intrinsic compressive stress in the range from 0.5 to 7 GPa [8, 9, 20]. The high internal stress limits the DLC films for various applications. Correlation of internal stress with sp3/sp2 bonded carbon and various properties have been studied by many authors [21-24]. While the correlation between surface structures and sp2/sp3 bonded carbon has not been investigated extensively |
III. EXPERIMENTAL SET-UP |
| Schematic diagram of the system is shown in Fig. 1. Vacuum chamber is made up of stainless steel (SS) having diameter 350 mm and height 400 mm. System is evacuated to high vacuum of 10-6 mbar by using the combination of rotary and turbo molecular pump. Operating pressure of the system is maintained to ~ 5×10-2 mbar by introducing acetylene and argon. The ratio of argon and acetylene is 1:1 (Total flow: 200 sccm). Mass flow controllers (maximum flow 100 sccm; Make MKS, USA) are used to control the flow of gases. For deposition of high quality, smooth and uniform DLC films with reproducible properties, the deposition must be performed in a high purity environment. Thus, experiments are carried out with 99.99% pure argon, hydrogen and acetylene. Circular power electrode of diameter 110 mm is used as the substrate holder. Air cooled RF generator (Model: RF-3XIII; Make: RFVII Inc. Williamstown, NJ) of 300 watt maximum power and 13.56 MHz is used for generation of plasma. Power is delivered through the automatic matching network (Make: PT-II-CE; Make: RFVII Inc. Williamstown, NJ) to the electrode. The coupling is done by special RF vacuum feed through. Counter electrode, the wall of the chamber, is grounded. Before DLC deposition, glass substrates are cleaned with deionized water, acetylene and isopropyl alcohol in ultrasonic vibrator and etched by Ar plasma for 10 minutes. During deposition no external heating is provided. All heating is provided by the plasma itself. |
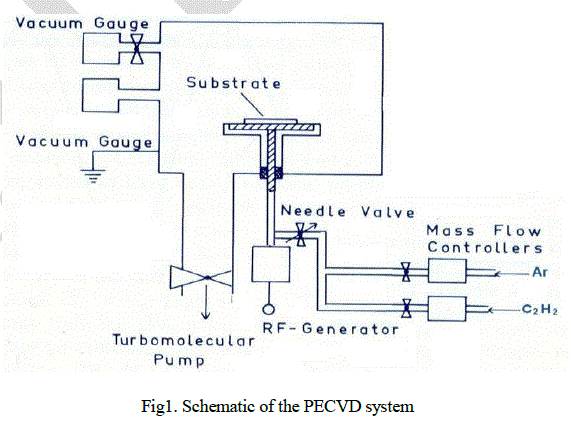 |
| Structural properties, hardness, surface morphology, roughness, and contact angle of the deposited films have been measured by Raman spectrometer, Nano indenter, Atomic Force Microscope, and Contact angle setup. Renishaw inVia Reflex Raman Spectrometer has been used for structural analysis. Measurements have been carried out at 100X magnification for 10sec exposure using 514 nm lasers. Spectra are taken at 50% laser power. Hardness is measured using UNMT-1 Universal Nano and Micro Tester, Center for Tribology, Inc., USA. Nanoindenter has been used for nano indentation. One mN load is used for indentation. Thickness of the films has been measured using Nano View Korea ellipsometer. Surface morphology has been studied by using NT-MDT Solver pro 47, Russia AFM. Contact angle of DLC films are measured with water using the OCAH 230 Data Physics, Germany contact angle measurement setup. |
IV. RESULTS AND DISCUSSION |
| DLC films are deposited with the variation of RF power, from 30 watt to 60 watt, on glass substrates at 5 × 10-2 mbar pressure. The films DLC 1, DLC 2, DLC 3, and DLC 4 correspond to 30 watt, 40 watt, 50 watt, and 60 watt RF power, respectively. Thicknesses of the films, measured by ellipsometer, are in between 650 nm to 700 nm. Raman spectra of DLC films are shown in Fig. 2, which confirm the formation of DLC films. Raman spectroscopy is a powerful technique for the identification of different phases, for the study of defects and structural disorders in thin films. It has the ability to distinguish between many possible-bonding geometries of carbon in carbon-based materials. One can get sufficient structural information of DLC films using Raman spectroscopy. Significant information can be obtained from G peak position, D peak positions and intensity ratio of D and G peaks. Correlation between various properties can be analyzed using Raman spectra [3, 25, 26]. |
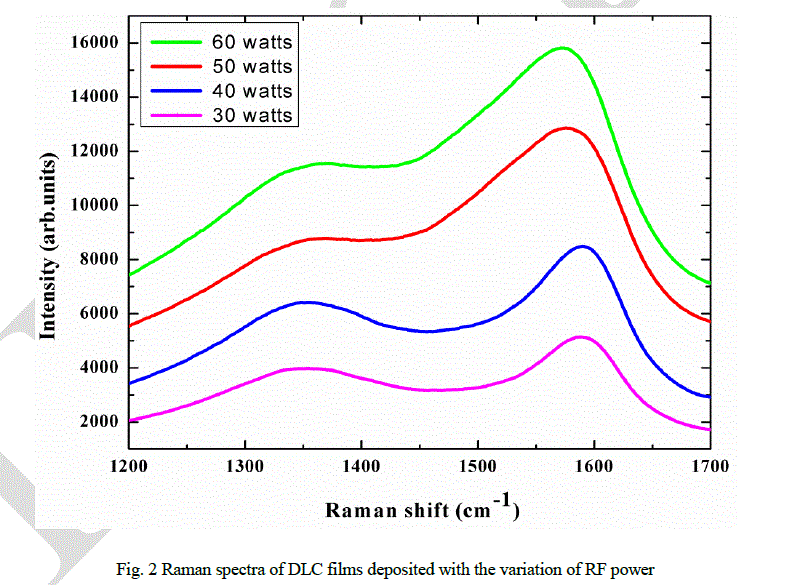 |
| It has been observed that the Raman spectra of DLC films (Fig. 2) have two predominant peaks centered around 1350 cm-1 and 1580 cm-1, known as D peak and G peak, respectively. Variation of I(D)/I(G) ratio and G peak and D peak positions with RF power are shown in Figs. 3 and 4, respectively. It has been observed that the ID/IG ratio decreases from 0.77 to 0.73, G- peak position shifts from 1588 cm-1 to 1576 cm-1 and D- peak position shifts from 1347 cm-1 to 1370 cm-1 with the increase in RF power from 30 watt to 60 watt. |
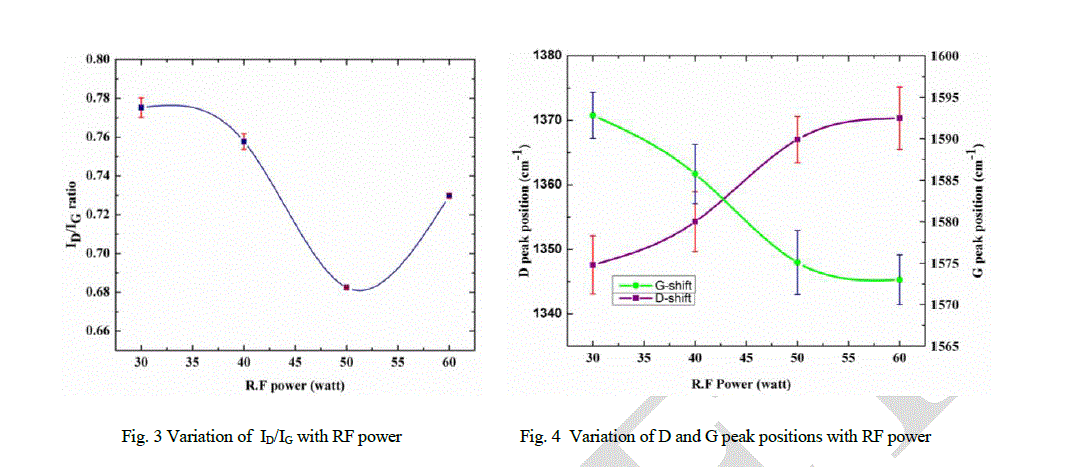 |
| Variation of the hardness with RF power is shown in Fig. 5. It has been observed that the hardness of the films increases (from 13.45 to 15.90 GPa) with RF power. |
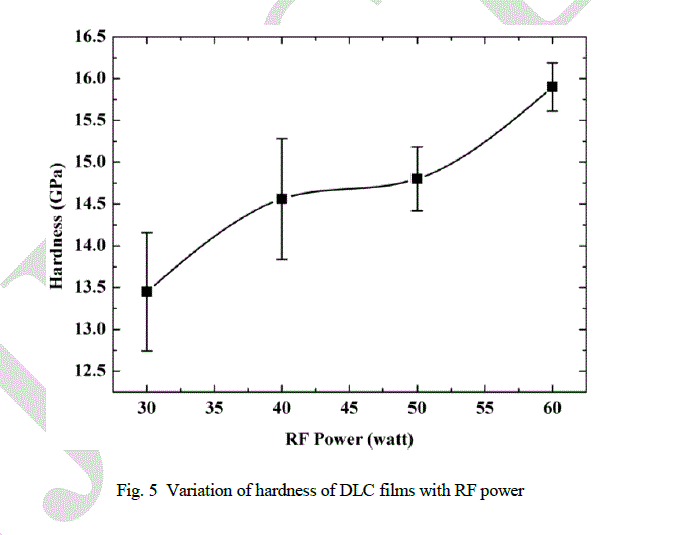 |
| Plot between indentation depth and load (maximum load 1 mN) is shown in Fig. 6. The curve shows the elastic behavior of the DLC films deposited at different RF power. It has been observed that the indentation depth of DLC 1 is more as compared to others and the behavior of DLC 1 is plastic as compared to DLC 2, DLC 3 and DLC 4. |
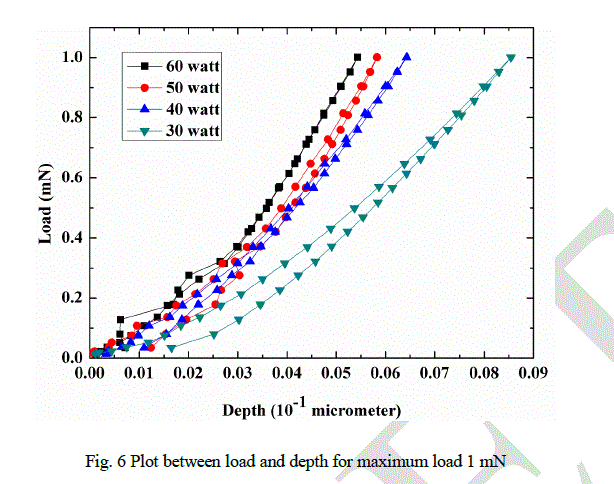 |
| Contact angle is another important surface property. In general, it is measured with liquid solid interface. It quantifies the wet-ability of the solid surface. Variation of contact angle with RF power is shown in Fig. 7. Contact angles show the hydrophobic nature of DLC films. It has been observed that as the RF power increases, contact angle decreases from 95° to 83°. It is maximum for the film deposited at 30 watt RF power. |
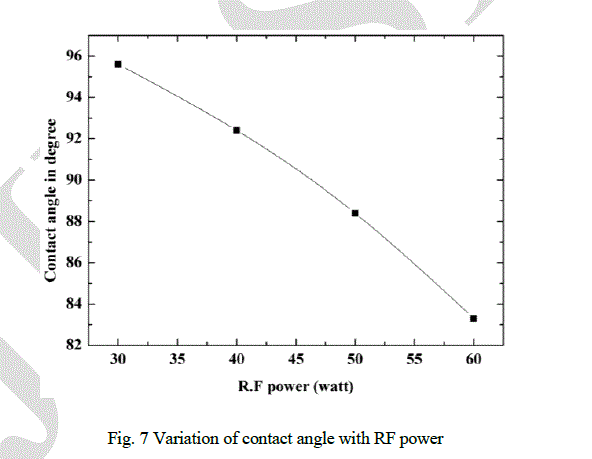 |
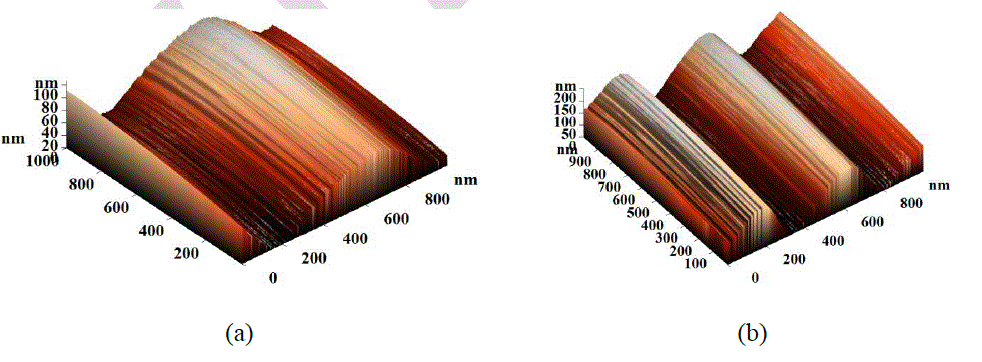
| Roughness of the DLC samples has been observed by using AFM. Plot between roughness (RMS and average) and RF power is shown in Fig. 8. It has been observed that the roughness of the films increases with RF power. The AFM images (three dimensional; Scan Area 1μm × 1μm) of DLC films are shown in Figs. 9 -12. The linear wave type morphology has been formed. It has been observed that the morphology varies with the variation of RF power. Formation of different surface morphologies like circular, granular, linear, and other structures have also been reported by many authors [21-24]. Formation of different structures depend on the process parameters like bias voltage, gas composition, type of power supply and power used. It has been observed that the structural and other properties are related with surface morphology of the films. |
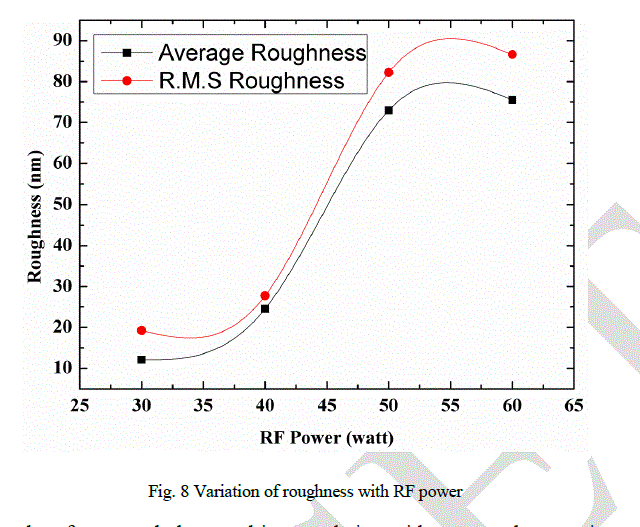 |
| Explanation of the observed surface morphology and its correlation with measured properties can be understood with the help of Raman spectra (Figs. 2, 3 and 4). The G peak position and I(D)/I(G) ratio indicate that more than 40% sp3 bonded carbon is present inside the films [3, 25, 26]. The observed hardness of all DLC films are more than 13 GPa which also indicate the presence of significant amount of sp3 bonded carbon in the films. The structure of the DLC film can be understood as the sp2 bonded carbon cluster in sp3 bonded amorphous matrix. The sp3 bonded carbon forms a network of C-C bond. Hydrogen is also associated with these sites. Significant hydrogen is available in hydrogenated DLC. Sp2 bonded carbon form small clusters in the matrix of sp3 bonded carbon. Sp3 bonded carbon matrix play an important role in determination of hardness as well as stress of the films. It can be argued that the surface morphology is also determined by the sp3 content in the films. It has been observed from AFM images that the surface morphology transforms from planer structure (Fig. 9a) to wave type structure (Figs. 9b, 9c and 9c) with the variation of RF power. Formation of wave structure can be understood with stress relax process in the films. |
 |
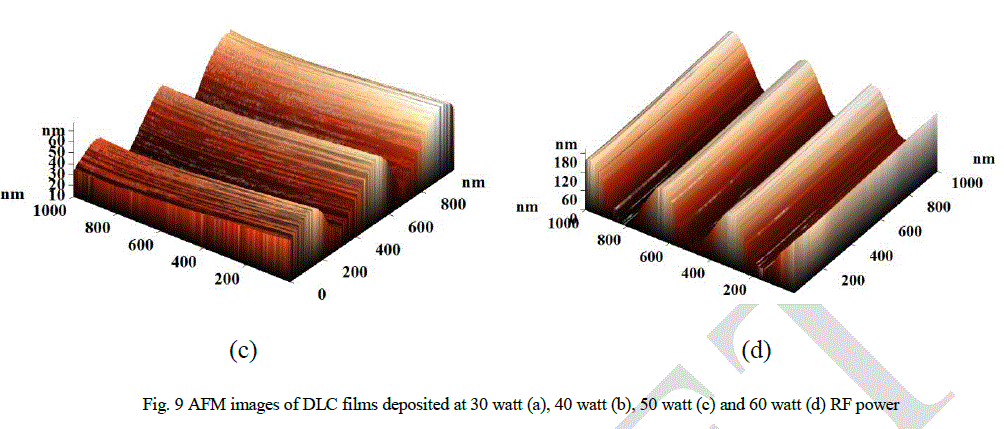 |
| Stable film can be formed when stress in the film is minimum. This can be done manually by introducing intermediate layers, post deposition heat treatment and by various pretreatments. Stress can also be relaxed naturally by structural change in sp3 bonded matrix. These structural changes are reflected in surface morphology as observed by the AFM images (Fig. 9). In present study, it has been observed that the self relax process produces a systematic wave pattern on the surface, which depends on the RF power. High value of stress up to 7 GPa in DLC films has been reported by many authors [8-10]. High value of stress restricts the thickness of the DLC films and is the main cause of peeling of the films from substrate, which also restricts the DLC films for many applications. Stress in DLC films depends on sp3 content which is related to the ion energy. Plot between load and depth (Fig. 6), variation of G peak position (Fig. 4) and hardness (Fig. 5) with RF power indicate the increment of sp3 bonded carbon with RF power. As RF power increases, the ion energy increases which promotes the formation of sp3 bonded carbon. In present investigation, all parameters like pressure, gas composition, gas flow etc. are kept constant, except RF power. Hence, the variation in ion energy is due to the variation in RF power. It can be argued that high ion energy produce high sp3 bonded carbon and hence high internal stress. From qualitative observation of the surface morphalogy, a conclusion can be drawn that smaller the wavelength of the wave structure higher will be the stress and vice-versa. The probability of peeling the films from the substrate is also reduced in complex morphology. Hence, from observations it can be concluded that the surface morphology with complex pattern has less stress and provides a stable structure compared to the smooth morphology. It can also be predicted that the formation of nano-clusters on the surface will also help to reduce the stress. |
| Two properties, contact angle and roughness are related to the surface and hence, depends mainly on the surface morphology. Stressed structure, due to high sp3 content, produce more roughness as reflected from Fig. 8. It has been observed that the contact angle decreases with the increase of RF power. It can be argued that the variation in contact angle is because of the change in surface morphology. It can be understood that the smooth surface shows good hydrophobic nature compared to the rough surfaces. However, the films with high roughness and complex surface structure will have mechanical properties like hardness. |
V. CONCLUSION |
| The diamond like carbon films have been prepared by PECVD method by argon and acetylene plasma at different R.F power. The positions of D and G peak in Raman spectra confirm the formation of DLC films. Variation of I(D)/I(G) ratio, G peak and D peak position with RF power provide significant structural information about the films. Raman spectra and hardness data confirm that the significant sp3 contents are present in the films. It can also be concluded from observations that sp3 content increases with RF power. It can be concluded that surface morphologies of the films are related to the stress, which depends on sp3 content of the films. Variation in morphologies are related to the stress relax process. Higher the stress, more complex surface morphology is generated. It can be concluded that the contact angle and surface roughness depend on surface morphology. Roughness is high for more complex stressed surface structure, contact angle is high for less complex structure. It can also be concluded that the deposited DLC films are hydrophobic in nature and smooth surfaces have greater contact angle compared to the rough surfaces. |
VI. ACKNOWLEDGEMENT |
| The authors are grateful to Department of Science and Technology (DST), Government of India for providing Raman Spectrometer under FIST program to BIT Mesra, Ranchi. |
References |
|