ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
|
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Modern dental restoration materials fulfil all the physical, biocompatibility and aesthetics requirements but they do not possess antibacterial activity to protect the tooth from recurrent caries. In this direction, many researchers worked on incorporation of different antibacterial agents like silver, antibiotics, Zinc oxide etc, in dental filling materials. The objective of present research work was to conduct the green synthesis of silver nanoparticles using Azadirachta indica extract and to incorporate these into dental filling material to confer antibacterial activity to the dental filling material. In order to fulfil the objective of this work, various experimental parameters of the process of green synthesis of silver nanoparticles were optimized. The SNPs were then characterized using UV-Vis spectrophotometer, particle size analyser, FTIR spectrophotometer. Their antibacterial activity was confirmed using agar diffusion test against E.coli and S.mutans. The silver nanoparticles were then incorporated into Glass Ionomer Cement (Fuji II) and the antimicrobial test conducted using S.mutans and the results indicated the increase antimicrobial property. The novel material is not soluble in the common beverages like tea, coffee, coca cola and lime juice
Keywords |
| Silver nanoparticles, Azadirachta indica, dental filling materials & green synthesis. |
INTRODUCTION |
| Silver nanoparticles can be synthesized using a number of approaches, for example, chemical approaches like reduction in solutions, thermal decomposition of silver compounds, radiation assisted, microwave assisted process and recently via green chemistry route using various bacteria like Pseudomonas stutzeri AG259 [10], Lactobacillus sp. A09 [11], fungi like F. oxysporum [12] natural products like green tea Camellia sinensis [13], leaf broth natural rubber [14] etc.. The use of environmentally benign materials like plant leaf extract, bacteria, fungi and enzymes for the synthesis of silver nanoparticles offers numerous benefits of ecofriendliness and compatibility for pharmaceutical and other biomedical applications as they do not use toxic chemicals for the synthesis protocol. [1-5,7] Green synthesis provides advancement over chemical and physical method as it is cost effective, environment friendly, easily scaled up for large scale synthesis and in this method there is no need to use high pressure, energy, temperature and toxic chemicals [15]. |
| Azadirachta indica commonly known as Neem belongs to Meliaceae family, and is well known in India and its neighboring countries for more than 200 years as one of the most versatile medicinal plant having a wide spectrum of biological activity. Every part of the tree has been used as a traditional medicine for household remedy against various human ailments, from antiquity [16]. |
| Dental caries, also known as tooth decay or a cavity, is an infection, bacterial in origin, that causes demineralization and destruction of the hard tissues of the teeth (enamel, dentin and cementum). The bacteria most responsible for dental cavities are most prominently Streptococcus mutans and Streptococcus sobrinus, and lactobacilli. The most common types of materials used for dental fillings are Amalgam, which is made of silver, tin, zinc, copper and mercury. Composite resins, which is made up of a mixture of plastic and fine glass particles; Cast Gold, which consists of gold alloy, that is, gold mixed with other metals; Ceramics, which are commonly made up of porcelain; Glass Ionomer, which is made up of acrylic and a component of glass called fluoroaluminosilicate. We have used Glass Ionomer as our control dental filling. The term secondary caries defines the process of caries development which occurs after treatment of the primary caries lesion. The prevalence of primary caries has been on decline worldwide since early 1980’s but secondary caries remains an unresolved problem in restorative dentistry. This necessitates the development of an antimicrobial dental filling material. The antimicrobial action is most often achieved by adding active antimicrobial ingredients to the dental material. In this study, silver nanoparticles are being investigated as an antimicrobial agent for their incorporation into denture materials. |
LITERATURE SURVEY |
| Silver has been used for its bactericidal properties for many years. It has been used in water purification, wound care, bone prostheses, reconstructive orthopedic surgery, cardiac devices and surgical appliances. The antimicrobial action of silver or silver compounds is proportional to the amount of released bioactive silver ions (Ag+) and its availability to interact with bacterial or fungal cell membrane. Nanoparticles are insoluble particles that are smaller than 100 nm in size. Their advantage is that they show stronger antimicrobial activity due to their higher surface area to volume ratio. Physical and chemical methods are more popular for nanoparticle synthesis but they remain expensive and the use of toxic compounds limits their application. An eco friendly green mediated synthesis of inorganic nanoparticles is a fast growing research in the limb of nanotechnology. Silver nanoparticles can be synthesized from bacteria, fungi and plants. Plants provide a better platform for nanoparticle synthesis as they are free from toxic chemicals as well as provide natural capping agents. Moreover, use of plant extracts also reduces the cost of microorganism isolation and culture media, enhancing the cost competitive feasibility over nanoparticle synthesis by microorganisms. Azadirachta indica plant extract is used for the green synthesis of SNPs in this study. Azadirachta indica leaf extract has also been used for the synthesis of silver, gold and bimetallic (silver and gold) nanoparticles. The major advantage of using the neem leaves is that it is a commonly available medicinal plant and the antibacterial activity of the biosynthesized silver nanoparticle might have been enhanced as it was capped with the neem leaf extract. The initial adhesion of specific oral bacteria to tooth surfaces is the primary and essential prerequisite for the development of cariopathogenic films. Within the complex formation of such biofilms, streptococcus mutans is primarily responsible for the initiation of tooth decay as well as for the progression of an established lesion. Ideally, the restorative materials should exhibit antibacterial properties to limit the adhesion and proliferation of pathogens at a very early stage and prevent secondary caries. Due to the few studies regarding the bactericidal effect of nanosilver particles and dental materials, the purpose of this study was to synthesize silver nanoparticles using green synthesis approach and to evaluate the antibacterial activity of glass ionomer dental cement modified by nanosilver particles. |
MATERIALS AND METHODS |
| Preparation of Azadirachta indica leaf extract and 1mM AgNO3 |
| Fresh leaves of Neem were collected and were washed thoroughly and allowed to dry. 25g of the dried leaves were washed thoroughly in distilled water. They were cut into fine pieces and taken in 100 ml sterile distilled water. The beaker was boiled over a Bunsen burner for 15 minutes. The mixture was allowed to cool to room temperature and the contents were filtered and stored at 4ᵒC. |
| 1mM Silver Nitrate solution contains 0.1699 g of AgNO3 in 1L distilled water. It was weighed and added to distilled water. |
| Optimization of Experiment Parameters |
| A set of experiments were conducted to first optimize the various experimental conditions. |
| The ratio of Neem leaf extract : 1mM AgNO3 solution was varied as: (1:2), (1:4), (1:6), (1:8) and (1:10). For the ratio (1:2), 10 ml of leaf extract was taken in a conical flask to which 20 ml of freshly prepared 1mM AgNO3 solution was added. The mixture was incubated in dark for 24 hours at room temperature. After 24 hours, the absorbance of the sample was determined within the range of 200-700 nm using UV-Visible spectrophotometer. The same procedure was repeated for all the ratios under study. The most optimum ratio of Neem leaf extract: 1mM AgNO3 solution (1:6) was then selected for further experiments. Neem leaf extract and freshly prepared 1mM AgNO3 solution were taken in the ratio of (1:6), in four conical flasks. The mixture was incubated in dark at room temperature for different incubation times: 2 hours, 5 hours, 24 hours and 48 hours and the absorbance of the sample was determined within the range of 200-700 nm, using UV-Visible spectrophotometer. The most optimum incubation time (24 hrs) was then selected for further experiments. Neem leaf extract and freshly prepared 1mM AgNO3 solution were taken in the ratio of (1:6), and were incubated in dark, for 24 hours at different temperatures, that is, at 10ᵒC, at 20ᵒC, at Room Temperature, at 30ᵒC and at 40ᵒC. After 24 hours, the absorbance of the sample was determined within the range of 200-700 nm using UV-Visible spectrophotometer. The incubation temperature which gave the best results (Room Temperature) was then applied for all further experiments. Neem leaf extract and freshly prepared 1mM AgNO3 solution were taken, in the ratio of (1:6) and were incubated in dark for 24 hours at room temperature with constant stirring and without stirring, respectively. After 24 hours, the absorbance of the samples was determined within the range of 200-700 nm using UV-Visible spectrophotometer. |
| Green Synthesis of silver nanoparticles |
| 50ml of Neem leaf extract was taken in a 500 ml conical flask. 300 ml of freshly prepared 1mM AgNO3 solution (1:6 ratio) was added to the flask. The flask was incubated in dark for 24 hrs at room temperature. |
| Collection of silver nanoparticles |
| The mixture obtained, after 24 hours, was centrifuged at 15000 rpm for 15 minutes. The supernatant was discarded and the pellet was retained in centrifuge tubes. The centrifuge tubes were kept in an oven, at 50ᵒC, overnight, to heat dry the pellet. Using a mini spatula, the dried pellet was scraped out and the SNP were collected. |
| Characterization of Silver Nanoparticles |
| The reduction of the Ag+ ions by the plant extract in solution and formation of SNPs were characterized by UV-Vis spectroscopy and the reduction of Ag+ ions was monitored by measuring the UV-Vis spectrum of the reaction medium between 200-700 nm range. The SNPs were dissolved in chloroform and then the particle distribution in liquid was studied in a computer controlled particle size analyzer. 2 ml of the sample was put into the machine and the readings and graph so obtained were recorded. The chemical composition of the synthesized silver nanoparticles was studied by using FTIR spectrometer. The phase variety and grain size of synthesized silver nanoparticles was determined by X-ray diffraction spectroscopy. The SNP sample is mounted on the sample holder and the results are recorded. The inoculums of E. coli and S. mutans were prepared in sterile nutrient broth and the flasks were incubated at 37ᵒC, overnight. Sterile nutrient agar plates were inoculated with the inoculums of E. coli and S. mutans by dipping a sterile cotton swab in the flasks and spreading the swab over the plate. 20μL of samples were put onto the disk. Ampicillin was used as a positive control, sterile distilled water and neem leaf extract as the negative control. Reaction medium containing silver nanoparticles were the sample under test and 1mM AgNO3 solution was used as a control. Zone of inhibition was measured after incubation. |
| Preparation of novel dental restoration material by the incorporation of SNPs |
| The material used for this study was conventional glass ionomer cement Fuji II. It is supplied as powder and liquid in separate bottles. Composition of the powder component is Fluoroaluminosilicate glass and of the liquid component is Copolymer of acrylic and maleic acids, polybase carboxylic acid, water. The powder liquid ratio (g/g) used is 2.7:1. The powder and liquid of Fuji II were mixed on a mixing pad as per the manufacturer’s instructions and were then packed into a mould. The material was allowed to set in the mould. This serves as a control sample for the further experiments. Then, 0.005 gm of SNPs, powder and liquid of Fuji II were again mixed on a mixing pad as per the manufacturer’s instructions as depicted in Figure 1 and were then packed into a mould. The material was allowed to set in the mould. This serves as the test sample for the further experiments. |
 |
| Antibacterial test on Novel product containing SNPs |
| Sterile nutrient agar plates were inoculated with the inoculums of S.mutans. Two cavities were made in the agar plate with a sterile copper coil, which were filled with the control cement and manipulated cement. Using a sterile spatula, two sterile filter paper disks were placed on the agar plates so that they were just touching the surface of agar. 20μL of Ampicillin (positive control) and 20μL of sterile distilled water (negative control) were put onto the disk. All plates were incubated at 37ᵒC for 24 hours. Zone of inhibition was measured after incubation. |
| Solubility test of GIC sample containing SNPs |
| The test media used for this study were tea, coffee, Coca-cola and lime juice. Four samples of the control and modified GIC were then immersed in 10ml of various test media at 37ᵒC for two days. The samples were again weighed. The solubility was determined using the formula: |
| Solubility = M1 – M2, where, M1 is the weight before immersion and |
| M2 is the weight after immersion. |
| An outline of the entire methodology is given in Figure 2 |
EXPERIMENTAL RESULTS |
| Biosynthesis of silver nanoparticles |
| Reduction of silver ions into silver nanoparticles during exposure to plant extracts was observed as a result of the color change. The color changes from light green to dark brown, which is indicative of silver nanoparticles synthesis [15][16][20] |
| The color change is due to the Surface Plasmon Resonance phenomenon. The metal nanoparticles have free electrons, which give the SPR absorption band, due to the combined vibration of electrons of metal nanoparticles in resonance with light wave. It was further confirmed by UV-Vis spectroscopy analysis. |
| UV-Vis Spectrophotometer Analysis |
| UV visible spectroscopy is one of the most widely used techniques for characterization of silver nanoparticles. The absorption spectrum of the brown color reaction medium obtained showed a surface plasmon absorption band with a maximum at 400 nm. From different literatures it was found that the silver nanoparticles show SPR peak at around 420 nm [15][16][20]. This indicates the presence of silver nanoparticles. |
| Optimisation of parameters |
| A. Neem leaf extract : 1mM AgNO3 solution ratio |
| Neem leaf extract and 1mM AgNO3 solution were taken in different ratios: (1:2), (1:4), (1:6), (1:8) and (1:10). Figure 4 depicts the results obtained on analyzing the samples using UV- Vis spectrophotometer. |
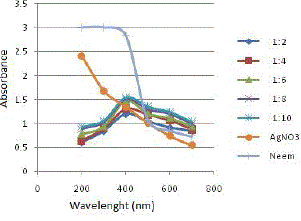 |
| All the samples showed absorption maxima at 400 nm, which confirms with the SPR peak of silver nanoparticles at 420 nm [15][16][20]. Table 1 gives the absorbance value of samples at 400 nm and the percentage increase in absorbance value from one ratio to another. |
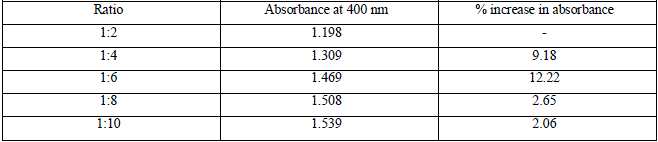 |
| As the amount of 1mM AgNO3 solution is increased in the reaction mixture, the absorbance value at the absorption peak of 400 nm also increases. However, the percentage increase in absorbance beyond the ratio (1:6) is very less. Thus, to maintain the cost effectiveness of the process of green synthesis of SNPs, (1:6) is chosen as the optimum ratio and is applied for all further experiments. |
| B. Incubation time |
| 4 different flasks containing the neem leaf extract and 1Mm AgNO3 solution in the ratio of (1:6) are taken and incubated for different incubation times: 2hrs, 5hrs, 24 hrs and 48 hrs. Figure 3 depicts the results obtained on analysing the samples using UV-Vis spectrophotometer. |
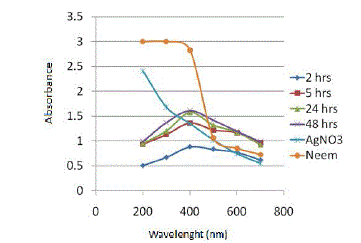 |
| All the samples showed absorption maxima at 400 nm, which confirms with the SPR peak of silver nanoparticles at 420 nm. Table 2 gives the absorbance value of samples at 400 nm and the percentage increase in absorbance value from one incubation time to another. |
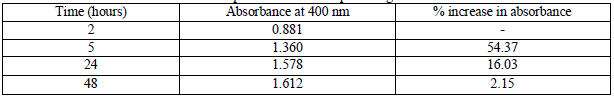 |
| As the incubation time of the reaction mixture is increased, the absorbance value at the absorption peak of 400 nm also increases. However, the percentage increase in absorbance value from 24 hrs to 48 hrs is very less (2.15%), thus for the ease of experimentation and to make the process of green synthesis of SNPs time efficient, the optimum incubation time is chosen as 24 hrs for all further experimentation. |
| C. Incubation temperature |
| The next experimental parameter to be optimized is the temperature at which the green synthesis of SNPs is to be conducted. The temperature is varied from 40C, 200C, room temperature, 300C and 400C. Figure 4 depicts the results obtained on analysing the samples using UV-Vis spectrophotometer. |
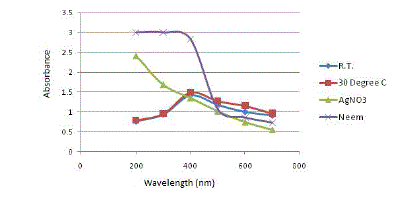 |
| All the samples showed absorption maxima at 400 nm, which confirms with the SPR peak of silver nanoparticles at 420 nm. Table 3 gives the absorbance value of samples at 400 nm and the percentage increase in absorbance value from one incubation temperature to another. |
 |
| With an increase in the incubation temperature, the absorbance value at the absorption peak of 400 nm also increases. The maximum absorbance value is obtained at 40 0C. However, the percentage increase in absorbance value from room temperature to higher temperatures like 30 0C and 40 0C is very less. Thus, to cut back on the cost of heating requirements, room temperature is chosen as the optimum temperature. |
| D. Stirring conditions |
| The next experimental condition to be investigated is whether green synthesis of SNPs requires continuous stirring or not. Figure 5 depicts the results obtained on analysing the samples using UV-Vis spectrophotometer. |
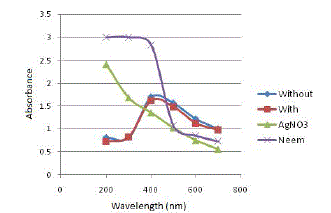 |
| All the samples showed absorption maxima at 400 nm, which confirms with the SPR peak of silver nanoparticles at 420 nm. Table 4 gives the absorbance value of samples at 400 nm and the percentage increase in absorbance value of sample incubated with stirring and without stirring. |
 |
| The reaction medium which was subjected to continuous stirring gives a higher absorbance value at 400 nm. However, the percentage increase in absorbance value is 5.12%. Since, the percentage increase in absorbance is not very significant and for ease of conduction of experiments and to reduce energy requirements of the process of green synthesis of SNPs, the further experiments were conducted without stirring. |
| Efficiency of conversion |
| 300 ml of 1Mm AgNO3 solution contains 0.051 gm of AgNO3 salt. |
| Atomic mass of Ag = 107.87 gm/mol |
| Molecular weight of AgNO3 = 169.87 gm/mol |
| This implies that 169.87 gm of AgNO3 salt contains 107.87 gm of Ag. |
| 0.051 gm of AgNO3 solution contains = (107.87 x 0.051) / 169.87 |
| = 0.0324 gm of Ag. |
| Mass of empty appendoff tube = 1.186 gm |
| Mass of appendoff tube containing collected SNPs = 1.2076 gms |
| Mass of SNPs collected = 0.0214 gm |
| Percentage of conversion of Ag = (0.0214 x 100)/ 0.0324 |
| = 66.05% |
| Characterization of Silver Nanoparticles |
| Particle size analysis results |
| According to the figure, the colloidal solution of silver nanoparticles of ratio 1:6 contains particles of different sizes ranging from 204.6 nm to 1204 nm. The average size of nanoparticles is 418 nm. The particle size distribution graph is depicted in Figure 6. |
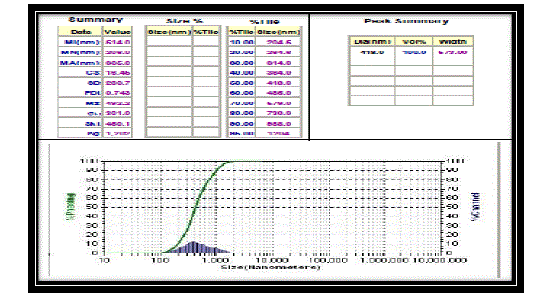 |
| FTIR results |
| FTIR measurements were carried out to identify the biomolecules for capping and efficient stabilization of the metal nanoparticles synthesized. Figure 7 gives the spectrum that was obtained on analysis of the sample in the range of 4000-550 cm-1. |
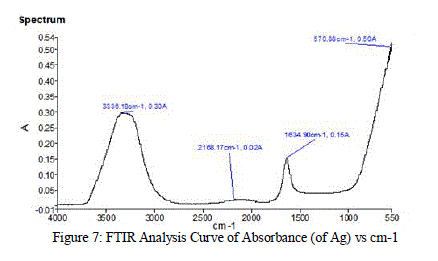 |
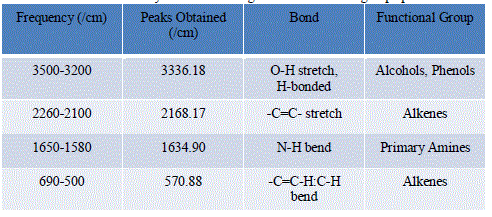
| The FTIR spectrum of silver nanoparticles showed the band between 3500-3200 cm-1 corresponds to O-H stretching and H-bonded alcohols and phenols. The peak found around 2260-2100 cm-1 showed a stretch for (-C=C-) bond which corresponds to alkenes, peak around 1650-1580 cm-1 showed the bond for (N-H) bend which corresponds to primary amines and peak around 690-500 cm-1 showed the bond for (-C=C-H:C-H) bend which again corresponds to alkenes |
| Comparing the peaks obtained in the FTIR analysis to the characteristic peaks, the various functional groups present in the sample were identified and tabulated in Table 5: |
 |
| Therefore the synthesized nanoparticles were surrounded by proteins and metabolites such as terpenoids having functional groups. From the analysis of FTIR studies we confirmed that the carbonyl groups from the amino acid residues and proteins has the stronger ability to bind metal indicating that the proteins could possibly from the metal nanoparticles (i.e.; capping of silver nanoparticles) to prevent agglomeration and thereby stabilize the medium. This suggests that the biological molecules could possibly perform dual functions of formation and stabilization of silver nanoparticles in the aqueous medium.Carbonyl groups proved that flavanones or terpenoids absorbed on the surface of metal nanoparticles. Flavanones or terpenoids could be adsorbed on the surface of metal nanoparticles, possibly by interaction through carbonyl groups or π-electrons in the absence of other strong ligating agents in sufficient concentration. The presence of reducing sugars in the solution could be responsible for the reduction of metal ions and formation of the corresponding metal nanoparticles. It is also possible that the terpenoids play a role in reduction of metal ions by oxidation of aldehydic groups in the molecules to carboxylic acids. |
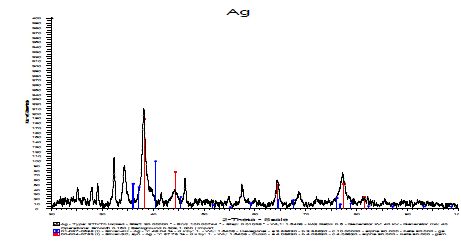
| XRD analysis |
| XRD spectrum showed distinct diffraction peaks around 380 angle. These sharp Bragg peaks might have resulted due to capping agent stabilizing the nanoparticle. Intense Bragg reflections suggest that strong X-ray scattering centres in the crystalline phase and could be due to capping agents. Broader peaks signify smaller particle size and reflect the effects due to experimental conditions on the nucleation and growth of the crystal nuclei. |
| The XRD analysis curve is a plot of the intensity, or counts as in this case, versus 2-Theta Scale. When the vertical representating a particular form of silver, corresponds to majority of the peaks obtained, that particular form of silver is said to be present in the sample under analysis. The sample under analysis showed the presence of 87.78% of Silver-3C as shown in Figure 8. |
 |
| Antibacterial testing analysis |
| A substance showing antibacterial activity will prevent the growth of microorganisms around it. Thus, when the substance is put on a disk placed on solid agar gel inoculated with microorganisms, it diffuses through the agar gel and prevents the growth of microorganisms around the disk. The diameter of this zone of inhibition is directly proportional to the antibacterial activity of a sample. Thus, greater the diameter of a zone of inhibition caused by a substance, greater is the antimicrobial activity that it possesses. |
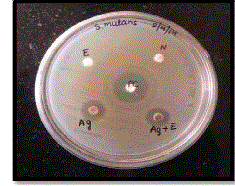
| The various samples used were denoted on plates as N-Negative Control (Distilled Water), E-Neem Leaf Extract, PCPositive Control (Ampicillin), Ag+E-Sample containing SNPs and Ag-1mM AgNO3 solution. The zone of inhibition produced by various samples against E.coli are shown in Figure 9. |
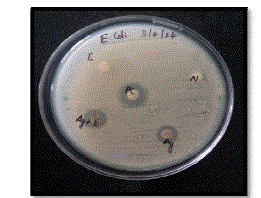 |
| The diameter of zone of inhibition given by various samples were measured and readings were tabulated in Table 6. |
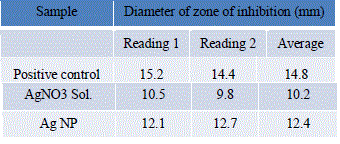 |
 |
| The percentage increase in the diameter of zone of inhibition of SNPs as compared to AgNO3 solution is 21.57%. Thus, there is a significant increase in the antibacterial activity of SNPs against E.coli. |
| The zone of inhibition produced by various samples against S.mutans are shown in Figure 10. |
 |
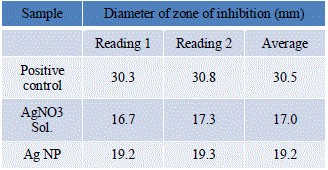
| The diameter of zone of inhibition given by various samples were measured and readings were tabulated in Table 7. |
 |
| The percentage increase in the diameter of zone of inhibition of SNPs as compared to AgNO3 solution is 12.94%. Thus, there is a significant increase in the antibacterial activity of SNPs against S.mutans. |
| Preparation of novel dental restoration material by the incorporation of SNPs |
| Addition of SNPs to Fuji II GIC was carried out. As aesthetics is one of the principle properties of an ideal filling material, the primary concern was that SNPs being dark in colour, might change the colour of GIC used, to gray or black. This would be a drawback as GIC normally give a tooth coloured product. However, as the amount of SNPs being added to GIC was less, there was no significant change in the colour of the GIC after addition of SNPs. |
| Antibacterial test of the new GIC product containing SNPs |
| Streptococcus mutans is a Gram-positive organism that is the primary causative agent in the formation of dental cavities in humans. Along with S. sobrinus, S. mutans plays a major role in tooth decay, metabolizing sucrose to lactic acid using the enzyme glucansucrase. S. mutans is one of a few specialized organisms equipped with receptors that improve adhesion to the surface of teeth. Thus the modified GIC containing SNPs should be able to exhibit antibacterial activity against S.mutans to prevent the adhesion of bacteria to tooth and to prevent the recurrence of secondary dental caries. |
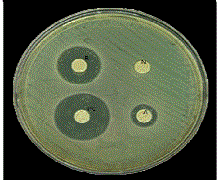
| The various samples used for agar diffusion test were A-Conventional GIC, B-GIC incorporating SNPs, N-Negative Control (Distilled Water) and PC-Positive Control (Ampicillin). The zone of inhibition produced by various samples against S.mutans is shown in Figure 11. |
 |
| The diameter of zone of inhibition given by various samples were measured and readings were tabulated in Table 8. |
 |
| The agar diffusion test shows that there is a significant increase (2.79 times) in the antibacterial activity of the GIC containing SNPs as against S.mutans, as compared to the conventional GIC. Thus, the addition of SNPs has conferred antibacterial activity to the GIC. |
| Solubility Test of the New GIC Product incorporating SNPs |
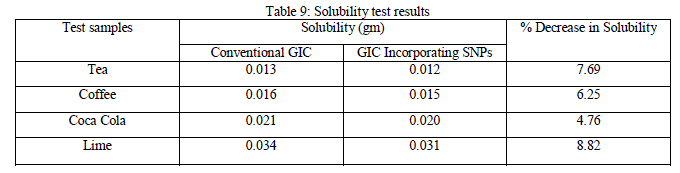
| One of the main factors that determine the durability of a material used in the oral environment is its chemical stability. Standard test of solubility as used in this study involves the storage of disc of materials in test media for a period of time, with the result being quoted as weight loss of the disc. The solubility results obtained were tabulated in table 4.9, along with the %decrease in solubility of the GIC containing SNPs as compared to conventional GIC. |
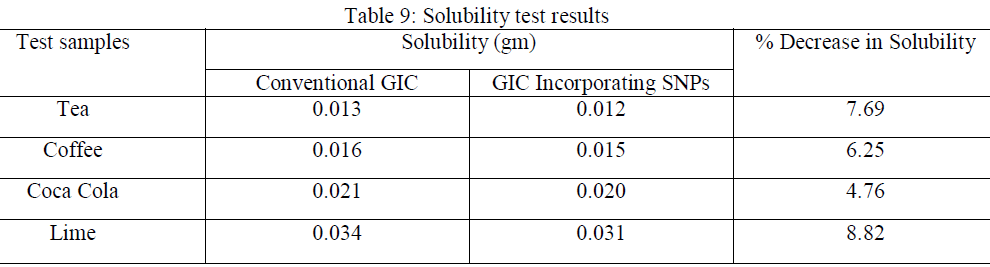 |
| There is not much change in solubility(<10%) from conventional GIC to GIC containing SNPs. |
| The material tested shows high values of solubility in lime juice. This result infers that lime juice is having an enhancing effect on these two properties than the other immersion media such as tea, coffee and coca cola. Maximum solubility is in lime and Coca Cola because lime contains Citric acid and Phosphoric acid is used in cola drinks. Citric acid was found to have more erosive potential on enamel and dentin. |
CONCLUSION |
| Green synthesis of SNPs using Azadirachta indica leaf extract was carried out optimally by taking Azadirachta indica leaf extract and 1mM AgNO3 solution in the ratio (1:6). The mixture was incubated in dark at room temperature for 24 hours without stirring. SNPs were successfully synthesized using Azadirachta indica leaf extract by the reduction of Ag+ ions in AgNO3 solution with a conversion efficiency of 66.5%. UV-Vis analysis of the sample gave an absorption maximum at 400 nm which confirmed the presence of SNPs as SNPs show an absorption maxima at 420 nm. The particle size analysis results showed that the SNPs synthezised had an average size of 418 nm. Further, ultrasonication of the sample reduced the particle size to nanometer size. XRD analysis indicated the presence of 87.78% of Silver-3C in the sample and the broad peaks of the graph indicate the presence of smaller size particles. In the case of E.coli, a 21.57% increase in the antibacterial activity of SNPs was obtained in comparison to 1mM AgNO3 solution. In the case of S.mutans, a 12.94% increase in the antibacterial activity of SNPs from AgNO3 solution was noted. This confirms that SNPs show greater antibacterial activity for both gram positive and gram negative bacteria. The new GIC product was successfully synthezised by incorporating SNPs without leading to any changes in the tooth-like appearance of the GIC. A 2.8-fold increase in the antibacterial activity against S.mutans of the manipulated GIC in comparison with the conventional GIC was succesfully achieved. Solubility tests of the conventional GIC and improved GIC confirmed a slight decrease (< 10%) in solubility of improved GIC. |
| Thus, the new GIC product incorporating SNPs proved to be better than the conventional GIC at antibacterial activity and solubility characteristics |