ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Muhamed Sajeer. K1, Muhammed Rafi. N2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Tin dioxide (SnO2) thin films have been deposited using chemical spray pyrolysis on non- conducting glass substrates at temperature 400oC with different solution concentration from 0.2M to 0.5M.The structural , optical and surface morphological properties of deposited films were studied using scanning electron microscope (SEM), Xray diffraction (XRD), UV-Vis spectrometry techniques. SEM studies reveal that SnO 2 films exhibited the irregular grains over the surface and the amount of grain increases with increase in the solution concentration. The XRD studies reveal that films are crystalline and structure of tetragonal phase. Micro structural parameters such as crystallite size, micro strain, and dislocation density are calculated and found to depend on solution concentrations. The optical band gap of SnO 2 thin films is calculated using transmittance and reflectance data using UV-Vis spectrometry. From the data, energy band gap lies between 2.72 eV to 2.95eV. And refractive index of the thin film is decreases with decrease in solution concentration
Keywords |
| Spray pyrolysis, SnO2 Thin films, SEM, XRD, UV-Vis spectrometry |
INTRODUCTION |
| Studies of dioxide films are quite important because of their possible technological applications. Among the various dioxide, tin dioxide (SnO2) is promising one and receiving ever-increasing attention owing to its potential uses in a wide variety of applications. It belongs to the II-VI family of semiconducting material owing to energy band gap value about 3 eV. This material is used as the transparent conductive coatings for liquid crystal displays, flat panel displays, plasma displays, touch panels, electronic ink applications, organic light- emitting diodes, solar cells, antistatic coatings and EMI shielding, gas sensing process and also for the fabrication of the optoelectronic devices such as blue-emitting diodes, electroluminescent devices, optical coating, n-window layers for thin film hetrojunction solar cells, photo conductor, photovoltaic devices. It is a good reflector and also dielectric filters due to the high refractive index and high transmittance in the visible range. Tin dioxide (SnO2) is the most important material for use in gas sensing applications. It is the dominant choice for solid state gas detectors in domestic, commercial and industrial settings due to the low operating temperatures, high sensitivities, mechanical simplicity of sensor design and low manufacturing costs. Tin dioxide is an n-type semiconductor, where the sensor conductivity increases in the presence of a reducing gases (such as CO), and decreases in the presence of an oxidizing gas (such as O2). SnO2 sensor response is due to surface interactions between the tin oxide and the surrounding gases [1]. |
MATERIALS AND METHODS |
| Several techniques have been used to produce SnO2 films such as thermal evaporation[2], spray pyrolysis[3], molecular beam epitaxy[4], sputtering[5], chemical bath deposition(CBD)[6-11]. Among them, the spray pyrolysis technique is particularly attractive because of its simplicity in comparison with required vacuum conditions or complex equipments. The method have been made to prepare SnO 2 thin films by simple and low cost chemical spray pyrolysis technique. In this work it is concentrated the growth and different characterisation of SnO2 thin film prepared by spay pyrolysis technique. The films have been characterized by X-ray diffraction (XRD), scanning electron microscope (SEM) and optical measurement techniques and the result have been discussed. |
EXPERIMENTAL |
| Various solution concentration of SnO 2 thin films are prepared using spray pyrolysis instrument by depositing onto glass substrates. The spray solution was prepared by mixing the appropriate quantity of SnCl 2.2H 2O dissolved in a mixture of methanol/ distilled water (volume ratio 9:1). A few drops of hydrochloric acid were added to increase the solubility of the solution. The hot substrate provides the thermal energy for the thermal decomposition and subsequent recombination of the constituent species. SnO 2 thin films of different molar concentrations (0.2 to 0.5 M) are prepared by keeping the constant substrate temperature (400 0C). |
| The structural characterization of the thin films was carried out by analyzing the XRD pattern obtained using Bruker AXSDS Advance X-ray diffractometer with Cu k α radiation ( λ = 0.154056 nm).Surface morphological was carried out using a scanning electron microscope ( JEOL Model, JSM-6390LV). Optical measurements were carried ot UV-Vis technique using instrument VARIAN make, model CARY 5000 with a spectral range of 200-1200 nm. |
RESULT AND DISCUSSION |
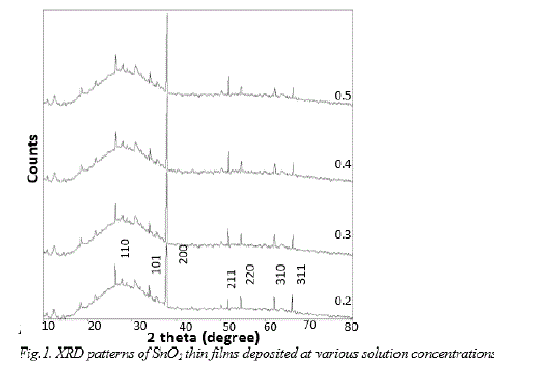
| X-ray diffraction pattern recorded for the spray deposited SnO 2 films on glass substrates at various solution concentrations from 0.2M, 0.3M, 0.4M, and 0.5M are shown in fig.1. It is observed that, in all the XRD pattern contains the characteristic SnO2 orientations along the preferred direction (200). The presence of other peaks such as (110), (101), (211), (220), (310) and (301) has also been detected but with substantially lower intensities. The intensity of (200) peak increases progressively as the concentration increases. The increase in (200) peak intensity may be attributed to the continuous increase in film thickness from 181.50 to 190.90 nm. At lower concentration (0.2 M), large number of small nuclei are formed at pyrolysis temperature 400ÃÂ C. The growth of each nucleus has taken place separately with different orientations and finally the crystal growth along (200) plane is dominant. With increasing solution concentration (0.2 to 0.5 M), initially formed large number of small nuclei might have agglomerated (coalescence) and relatively small number of large nuclei might have formed, which might have caused minor peaks to suppress or disappear and growth along (200) plane is further dominated. |
 |
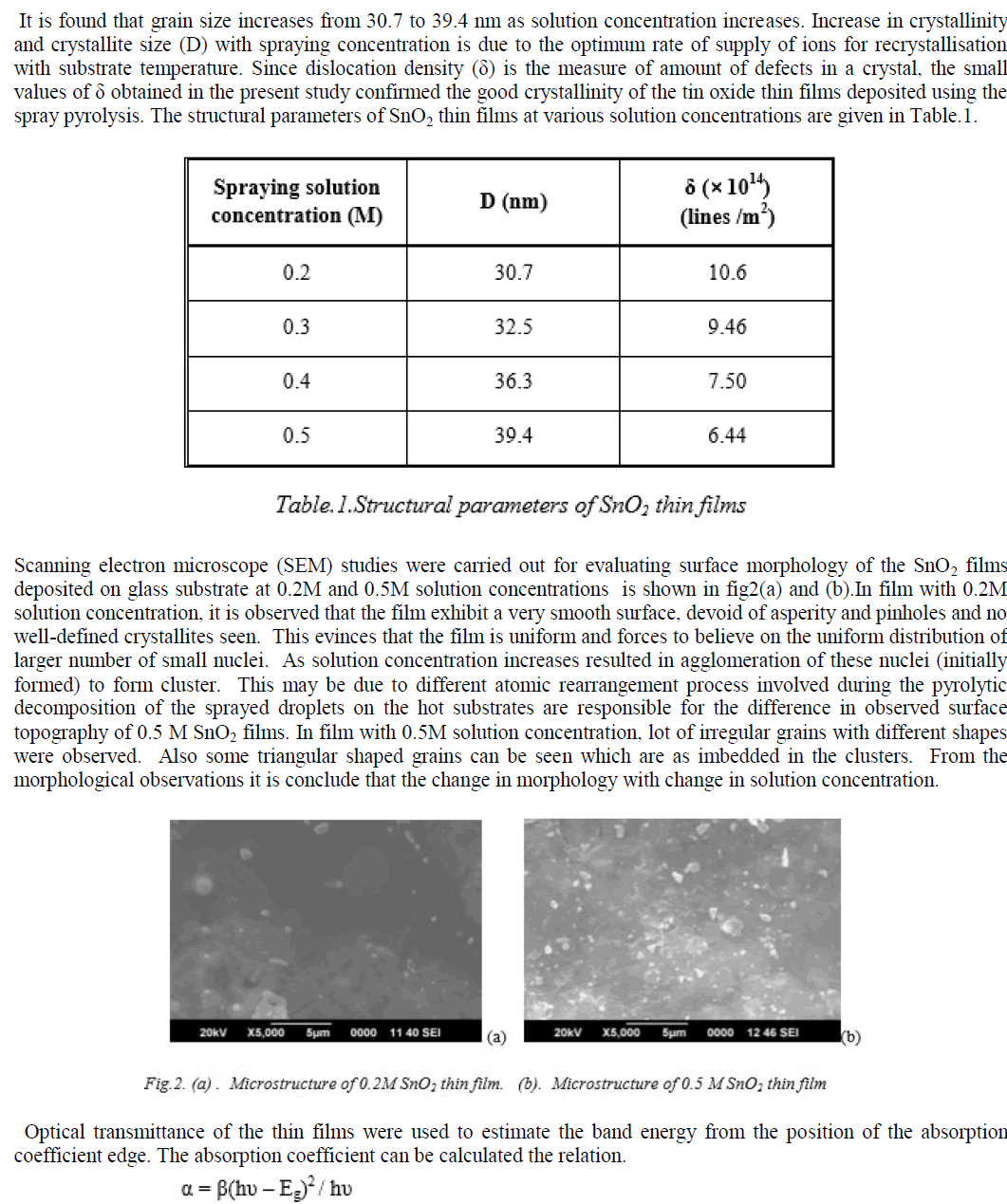 |
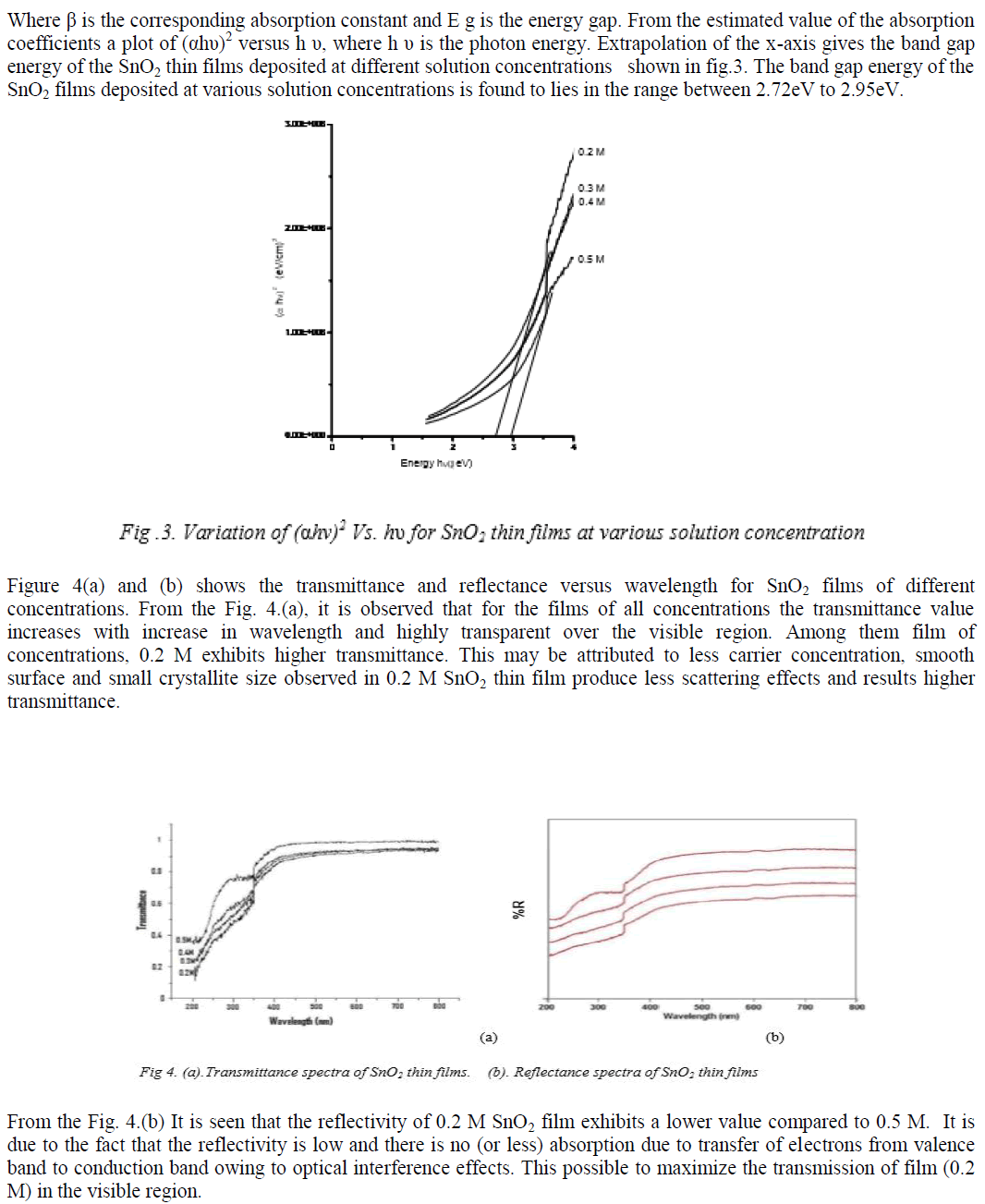 |
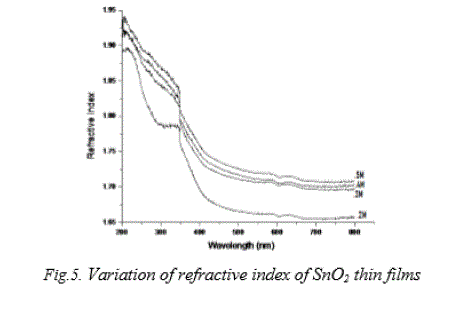
| Refractive index for tin dioxide thin films were evaluated from the transmittance and reflectance measurements and its variation with wavelength are shown in Fig. 5. From the plot, it is clear that the refractive index decreases with decrease in solution concentration. For all films, the refractive index is found to decrease in the wavelength region 200 nm to 450 nm and thereafter remains more or less constant. The refractive index lies between 1.68 and 1.89 for 0.2 M SnO2 films and 1.73 and 1.94 for 0.5 M SnO2 films. This may be due to solution concentration, mobility and coarse surface effect of thin films. |
 |
CONCLUSION |
| The SnO2 thin films were successfully deposited on glass substrate at various solution concentrations 0.2M, 0.3M, 0.4M and 0.5M using spray pyrolysis technique. X-ray diffraction analysis confirmed that the deposited SnO2 films were crystalline and tetragonal structure. Various structural parameters such as crystalline size, dislocation density are calculated and found to depend upon various solution concentrations. SEM studies reveal that irregular grains were increased with respect to the increase in solution concentration. The surface morphology of the thin films changes with change in solution concentrations. Optical transmittance measurements indicate that the deposited thin films have band gap energy lies in the range between 2.72 eV to 2.95eV which confirm these are good semiconducting films. |
References |
|