ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
G.Valli1 & M.Murugalakshmi2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Knowing the importance of Manjanathi fruit called as Indian Noni as extensively revealed by various scientific groups, our study focus on isolation of bioactive constituents of ethanol extract of Manjanathi fruit(EEMF) collected from Rajapalayam area of Virudhunagar district of Tamilnadu. Preliminary Phytochemical analysis revealed the presence of Alkaloids, Carbohydrates, Proteins, Amino acids, Phenols, Flavonoids, Glycosides, Anthraquinone glycosides, Coumarin glycosides, Oils and fats. Phenols ,Alkaloids,and Flavonoids were found to be present in moderately more in quantity. Agar well method was adopted for the determination of antibacterial activity, against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Pneumococcus pneumoniae, Klebsiella pneumoniae, Streptococcus Pyogens, Salmonella paratyphi , Citrobacter, Proteus vulgaris and Bacillus subtilis. . Compared to standard Ciproflaxcin, EEMF possessed higher activities against Streptococcus pyogenes (6mm) and Pseudomonas aeruginosa (10mm) and closer activity against Klebsiella pneumoniae(8mm) as that of the standard Ciprofloxacin. One Compound was separated from other constituents by column chromatographic technique using 60% Methyl acetate: 40% Chloroform solvent mixture, was found to have the molecular formula C9H18O.The compound was characterized by spectral techniques like Fourier Transform Infrared spectroscopy,Nuclear Magnetic Resonance spectra-1H NMR and 13C NMR. FT – IR Spectrum of isolated compound at 3394 cm-1 indicated presence of –OH group and band at 2975.96 cm-1 indicate the presence of (CH3- CH-) groupings. Absorption range from 1120 cm-1 -1030 cm-1 was due to (C-O) stretching. 1H NMR spectral studies revealed that a Singlet at 4.89 ppm is due to the -OH proton. A Multiplet at 3.69 ppm is due to the (-CH2- CH2-) grouping equivalent to 10 protons. A Septet at 1.233 ppm in due to presence of – CH – (CH3)2 group and a Doublet of 1.140 ppm due to CH3-CH- group. From the above datas , the compound was found to be 4-Isopropyl cyclohexanol.
Keywords |
| Manjanathi fruit ,Agar well method, 4-Isopropyl cyclohexanol and Ciprofloxacin. |
INTRODUCTION |
| There are several plants families known for their medicinal properties found in India. Several elderly women in Tamil Nadu find plant parts useful in curing Mantham, Digestive disorders, especially in children, Gastropathy, Dyspepsia, Diarrhea, Ulcerative Stomatitis, Wounds, Gout, Inflammation, Hernia, Dysentery and Fever. We have selected Manjanathi fruit for our project work. Manjanathi called as indian Noni is a popular medicinal plant among the siddha practitioners of Tamilnadu. The genus Morinda (Rubiaceae) including the species Morinda citrifolia is made up of around 80 species. Different parts of the plant including stem, bark, root, leaf and fruits have been used in the system of traditional medicine to treat a broad range of diseases including hypertension, [1, 2] arteriosclerosis, [3] colic and diarrhoea. Morinda citrifolia has been reported to possess antithrombotic, [4] antioxidant, [5] analgesic, anti-inflammatory [6] and xanthine oxidase inhibitory [7] activities. There are also preliminary studies reporting its blood pressure lowering and vasodilatory properties. [8]. The chemistry of noni has been investigated extensively by various scientific groups. A plethora of phytochemical constituents have been identified in the leaves, bark, and stem, flowers and fruits of the plant. Noni fruit specifically is a rich source of phytochemical constituents which demonstrate bioactivity. |
II.EXPERIMENTAL WORK |
| A. Isolation of Compounds from Manjanathi Fruit: |
| The fresh unriped fruits (Figure-I & II) of Manjanathi were collected from Thevathanam and Rajapalayam area of Virudhunagar district of Tamilnadu and washed with water to remove any adhering foreign particles and soil materials and then the fruit was made to undergo ripening as the collection of riped fruit was found to be difficult for us.. These were dried under shade and crushed well to remove the seeds and then extracted with ethanol followed by filtration. The filtered extract was refluxed for 3 hours in a soxhlet apparatus. The filtrates were then evaporated to give a dark colored viscous mass and on cooling produced solid mass. |
 |
 |
B. Preliminary Phytochemical studies: |
| Preliminary phytochemical analysis was carried out for the ethanol extract of Manjanathi fruit (EEMF). The chemical tests for Alkaloids, Carbohydrates , Phenols , Flavonoids Proteins , Amino acids, Steroids, Glycosides and Oils and fats were carried out to identify the presence of various Phytochemical constituents according to the standard procedures. The various Phytochemical constituents present were listed in the following Table-I. |
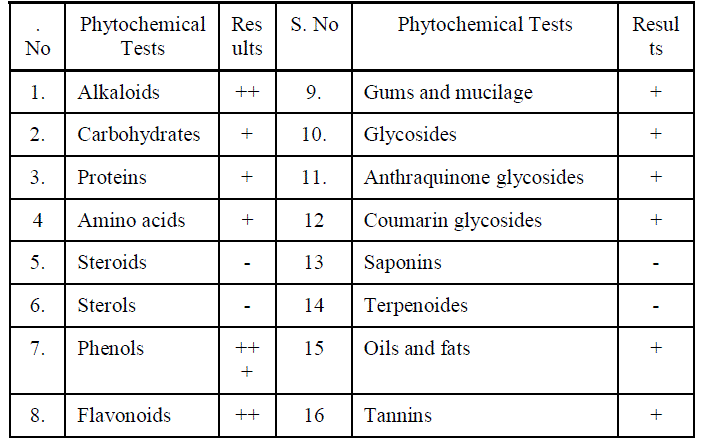 |
 |
C. Antibacterial Activity Determination: |
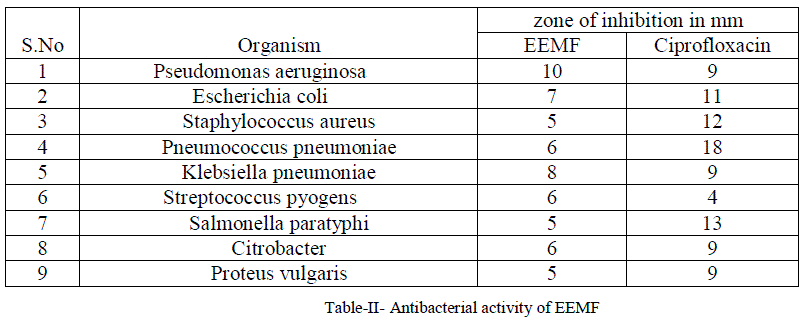
| For the antibacterial activity determination, Agar well method was adopted and the plate was run for 24 hours. The observed antibacterial activity of compound isolated from Manjanathi fruit extract against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Pneumococcuspneumoniae, Klebsiella pneumoniae, Streptococcus Pyogens, Salmonella paratyphi , Citrobacter, Proteus vulgaris, Bacillus subtilis compared with the standard drug Ciprofloxacin were listed in Table-II |
III. IDENTIFICATION OF BIOACTIVE COMPOUND |
| The number of compound in the extract can be identified by using Thin Layer Chromatography (TLC) Technique.we observed that there was one only clear spot is obtained in the 60% Methyl acetate: 40% Chloroform solvent mixture as given in Figure-1II. |
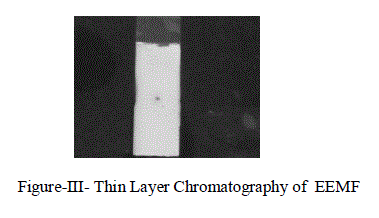 |
| Characterisation of Compound: The above obseved Compound was separated from other constituents by column chromatographic technique using 60% Methyl acetate: 40% Chloroform solvent mixture . From Elemental Analysis the molecular formula of the compound as given in Figure-IV was found to be C9H18O and was characterized by spectral techniques like Ultra violet spectrometry, Fourier Transform Infrared spectroscopy., Nuclear Magnetic Resonance spectra-1H NMR., 13C NMR |
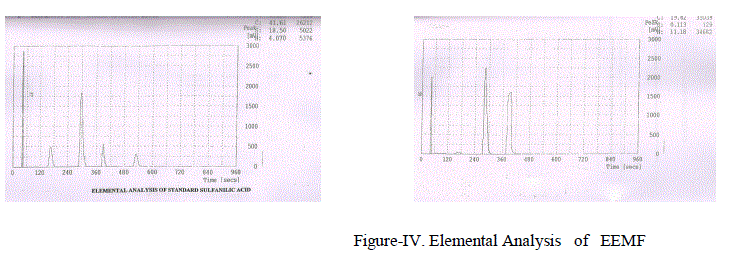 |
| ( i) UV- Spectral Studies: UV Spectrum was recorded in Elico (Thermo Spectronic ~ Visionpro Software VI.06) UV Spectrum of the above compound showed a absorption band at 399 nm by using chloroform as a reference solvent . (ii) FT – IR Spectral Studies: FT-IR characterization was done using SHIMADZU FT-IR 8400S. Fourier Transform Infrared Spectro Photometer by KBr pelletisation method. FT – IR Spectrum of isolated compound(Figure-V) have shown the following characteristic IR absorption frequencies.Broad band at 3394 cm-1 due to presence of –OH group.The band at 2975.96 cm-1 indicate the presences of (CH3-CH-) stretching frequency.Absorption at 2927.74 cm-1(-CH2-) stretching. 2894.95 cm-1 due to (-CH-) stretching. Absorption range from 1120 cm-1 -1030 cm-1 due to (C-O) stretching. |
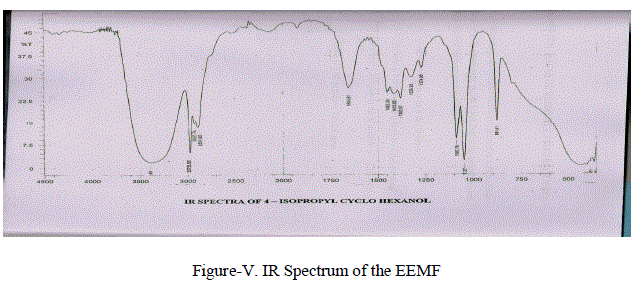 |
| (iii) Proton Magnetic Resonance Spectra-1H NMR. Nuclear magnetic resonance spectra were recorded on a Perkin-Elmer R32 (400 MHZ) spectrometer in CDCl3 with TMS as the internal standard(Figure-VI) and the chemical shifts were given in –δ value. A Singlet at 4.89 ppm is due to the -OH proton.A Multiplet at 3.69 ppm is due to the (-CH2- CH2-) grouping equivalent to 10 protons.A Septet at 1.233 ppm in due to presence of – CH – (CH3)2 group A Doublet of 1.140 ppm due to CH3-CHgroup. |
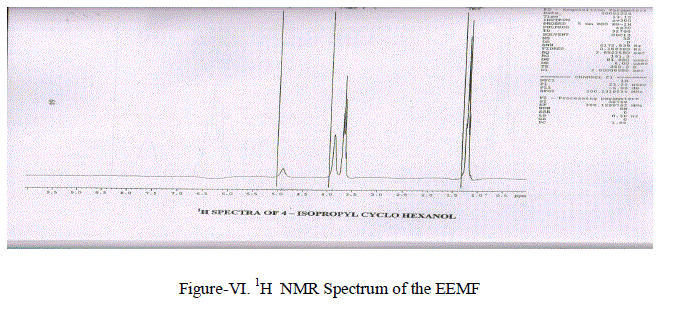 |
| (iv) 13C NMR Spectra: 13C NMR Spectrum (Figure-VII) showed the following Chemical shift for the Carbon atoms 77.04 δ corresponds to C1. 34.02 δ, 28.80 δ, 28.80 δ and 24.07 δ are due to (-CH2-) groups.24.713 δ may be due to (-CH-) group. 16.942 δ and 22.43 δ Corresponds to (-CH3-) groups. |
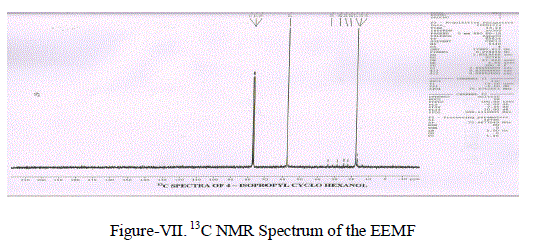 |
IV. RESULT AND DISCUSSION |
| The ethanol extract of Manjanathi fruit showed moderate activity against most of the micro organisms selected for the determination of antibacterial Activity. Compared to standard Ciproflaxcin, EEMF possessed higher activity against Staphylococcus pyogenes (6mm) and Pseudomonas aeruginosa (10mm) and closer activity against Klebsiellapneumoniae as that of the standard Ciprofloxacin(Figure-VIII). |
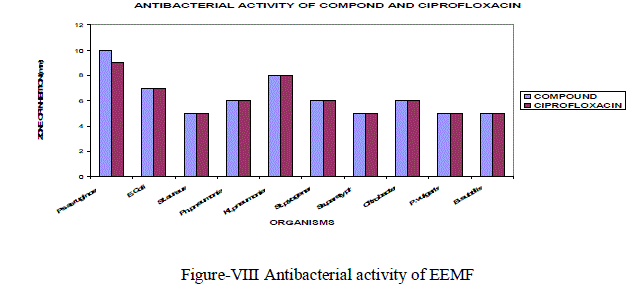 |
| Separation of constituents by column chromatographic technique using 60% Methyl acetate: 40% Chloroform solvent mixture yield one compound. That compound was found have the molecular formula C9H18O and was characterized by spectral techniques. FT – IR Spectrum of isolated compound at 3394 cm-1 indicate presence of –OH group and band at 2975.96 cm-1 indicate the presences of (CH3-CH-) groupings. Absorption range from 1120 cm-1 -1030 cm-1 due to (C-O) stretching. From 1H NMR spectral studies reveals that a Singlet at 4.89 ppm is due to the -OH proton. A Multiplet at 3.69 ppm is due to the (-CH2- CH2-) grouping equivalent to 10 protons.A Septet at 1.233 ppm in due to presence of – CH – (CH3)2 group and a Doublet of 1.140 ppm due to CH3-CH- group. From the above data the tentative structure of the compound may be |
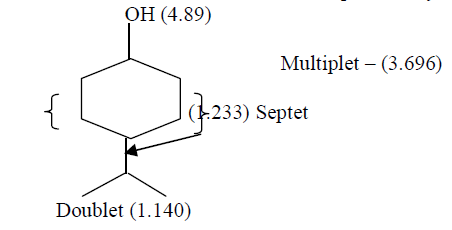 |
| 13C NMR Spectral values showed the following Chemical shift for the Carbon atoms δ 77.04 corresponds to C1.δ34.02, δ28.80, δ28.80 and δ24.07 were due to (-CH2-) groups. δ24.713 may be due to (-CH-) group.δ 16.942 and δ 22.43 Corresponds to (-CH3-) groups. From all the above studies, we concluded that the compound isolated from One of the component present in EEMF was found to be4-Isopropyl cyclohexanol. |
V.CONCLUSION |
| Bioactive constituents of Manjanathi fruit(EEMF) were extracted using ethanol by soxhlet extraction method .Preliminary Phytochemical analysis revealed the presence of Alkaloids, Carbohydrates, Proteins, Amino acids, Phenols, Flavonoids, Glycosides, Anthraquinone glycosides, Coumarin glycosides, Oils and fats. Phenols ,Alkaloids,and Flavonoids were found to be present in moderately more in quantity. Agar well method was adopted for the determination of antibacterial activity, against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Pneumococcus pneumoniae, Klebsiella pneumoniae, Streptococcus Pyogens, Salmonella paratyphi , Citrobacter, Proteus vulgaris and Bacillus subtilis. . Compared to standard Ciproflaxcin, EEMF possessed higher activities against Streptococcus pyogenes (6mm) and Pseudomonas aeruginosa (10mm) and closer activity against Klebsiella pneumoniae(8mm) as that of the standard Ciprofloxacin. One Compound was separated from other constituents by column chromatographic technique using 60% Methyl acetate: 40% Chloroform solvent mixture, was found to have the molecular formula C9H18O.The compound was characterized by spectral techniques like Fourier Transform Infrared spectroscopy,Nuclear Magnetic Resonance spectra-1H NMR and 13C NMR. FT – IR Spectrum of isolated compound at 3394 cm-1 indicated presence of –OH group and band at 2975.96 cm-1 indicate the presence of (CH3-CH-) groupings. Absorption range from 1120 cm-1 -1030 cm-1 was due to (C-O) stretching. 1H NMR spectral studies revealed that a Singlet at 4.89 ppm is due to the -OH proton. A Multiplet at 3.69 ppm is due to the (-CH2- CH2-) grouping equivalent to 10 protons. A Septet at 1.233 ppm in due to presence of – CH – (CH3)2 group and a Doublet of 1.140 ppm due to CH3-CH- group. From the above datas , the compound was found to be 4-Isopropyl cyclohexanol. |
ACKNOWLEDGEMENT |
| The authors express his sincere thanks to the College Management, Principal of SFR College, Sivakasi for providing necessary research facilities and also to Prof. P.Solairaj and Prof. A.Thanga Thirupathi of SB College of Pharmacy Anaikuttam, for Pharmacological activity determinations. The authors also thank the University Grants Commission (UGC), New Delhi for financial assistanc. |
References |
|