ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K.BALASUBRAMANI1, N.SIVARAJASEKAR2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Activated carbon was developed from coconut shell, characterized and used for the removal of Rhodamine-B from wastewater successfully. The methylene blue number, iodine number and BET surface area of the prepared carbon were found to be 80 mg g-1, 600 mg g-1, and 1200 m2 g-1 respectively. Rhodamine B is one of the water soluble, basic red cationic xanthene class dyes, a common water tracer fluorescent. Adsorption studies were carried out using Rhodamine B at different concentrations (150-400 mg g-1), pH (2-12), and adsorbent dose (1-3 g l-1). The adsorption data were correlated with both Langmuir and Freundlich models. The results indicated that the Freundlich model fitted the data better as compared to the Langmuir model .
Keywords |
| Rhodamine B, Activated carbon, Isotherms, Adsorption. |
INTRODUCTION |
| In recent years, pollution from dye wastewater has become a serious environmental problem due to the vast and increasing uses of a variety of dyes. Dyes are widely used in industries such as textiles, rubber, paper, plastics, cosmetics, etc., to colour their products. The dyes are invariably left as the major waste in these industries. Due to their chemical structures, dyes are resistant to fading on exposure to light, water and many chemicals and, therefore, are difficult to be decolourised once released into the aquatic environment (Sharma et al,2008). Many of the organic dyes are hazardous and may affect aquatic life and even the food chain. Release of these dyes in water stream is aesthetically undesirable and has serious environmental impact. Due to intense color they reduce sunlight transmission into water hence affecting aquatic plants, which ultimately disturb aquatic ecosystem; in addition they are toxic to humans also. In an attempt to solve dye pollution problems, methods like reverse osmosis, membrane separation, coagulation, chemical oxidation, biological treatments, photo degradation and adsorption have been used; but the most efficient method has been through adsorption process. Adsorption systems are rapidly gaining prominence as treatment processes that produce good quality effluents that are low in concentration of dissolved organic compounds, such as dyes. Regarding the selection of adsorbents, a literature survey shows that materials such as commercially available activated carbons, zeolites, etc., have been used in the past for the treatment of textile effluents. Despite the prolific use of activated carbon for the wastewater treatment, carbon adsorption remains an expensive process, and this fact over recent years has prompted a growing research interest into the production of low-cost alternatives to activated carbons. Various low-cost materials have been used for the removal of dyes from time to time. Such materials range from industrial waste to agricultural products. Various workers have exploited substances such as peat, bagasse pitch, Fuller’s Earth, lignite, coal, activated slag, activated carbon developed from fertilizer waste, bagasse fly ash, activated carbon fibers, wool carbonizing waste, clays, perlite, silica, wood meal, activated carbon developed from bamboo, alum sludge, and fly ash for this purpose. Coconut shell is an agricultural solid waste, available throughout the year. We made an attempt to utilise an adsorbent from this low cost material for dye removal from aqueous solution. Rhodamine B (RhB) is one of the water soluble xanthenes class dyes, a basic red cationic dye which is a common water tracer fluorescent. It is often used textile and food industries. It was an model pollutant to understand the adsorption capacity of the coconut shell carbon. This study is aimed at using a real world environmental process column study which requires little operator attention, easy inspection and cleaning for regeneration of adsorbent. |
MATERIAL AND METHODS |
| 2.1 Materials |
| Rhodamine B, is an cationic dye having IUPAC name as 9-(2-carboxyphenyl)-6-diethylamino-3-xanthenylidene]- diethylammonium chloride and it was procured from S-D fine chemicals Pvt. Ltd, Mumbai and Coconut shell activated carbon was obtained from water system Pvt. Ltd, Villupuram. The stock solution was prepared in double-distilled water by dissolving 1g dye in it. All the test solutions were prepared by diluting the stock with double- distilled water. |
| 2.2 Batch Equilibrium method |
| The known weight of adsorbent was added to 100 ml of the dye solutions with an initial concentration of 150 mg L-1 to 350 mg L-1. The contents were shaken thoroughly using a mechanical shaker rotating with a speed of 120 rpm. The solution was then filtered at preset time intervals and the residual dye concentration was measured by double beam UVVisible spectrophotometer 548nm filters. The amounts of dye adsorbed () and percent of removal (%R) can be calculated using the following equations: |
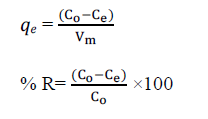 |
| Where qe is the amount of dye adsorbed (mg g-1). C and C are the initial and equilibrium liquid-phase concentrations of dye (mg g-1), respectively. V is the volume of the solution (l), and m is the weight of the sorbent used (g). |
EFFECT OF VARIABLE PARAMETERS |
| 3.1 Contact time |
| The effect of contact time on the removal of dye by the adsorbent was determined by keeping particle size, initial concentration, dosage, pH and concentration at 150 mg L-1, 1grm, 7pH and 300C, respectively. |
| 3.2 pH |
| Adsorption experiments were carried out at pH 4, 7 and 12. The acidic and alkaline pH of the media was maintained by adding the required amounts of 0.1N hydrochloric acid and sodium hydroxide solutions. The parameters like particle size of the adsorbents, dye concentration, dosage of the adsorbent and contact time were kept constant at while carrying out the experiments. The pH of the samples was determined using a portable pH meter (Systronics Model). |
| 3.3 Initial concentration of dye |
| Experiments were conducted with different initial concentrations of dyes ranging from 150 to 400 mg L-1. All other parameters were kept constant such as constant time, pH, temperature, adsorbents dosage were fixed at 3hrs, 7pH, 1grm and 300C, respectively. |
| 3.4 Dosage of adsorbents |
| The various doses of the adsorbent 1,2,3 (mg L-1) were mixed with the dye solutions and the mixture was agitated in a mechanical shaker. Other parameter such as initial concentration, contact time, pH, temperature at 150mg L-1, 3hr, 7pH and 300C, respectively. |
RESULTS AND DISCUSSION |
| 4.1 Characterization of the adsorbent |
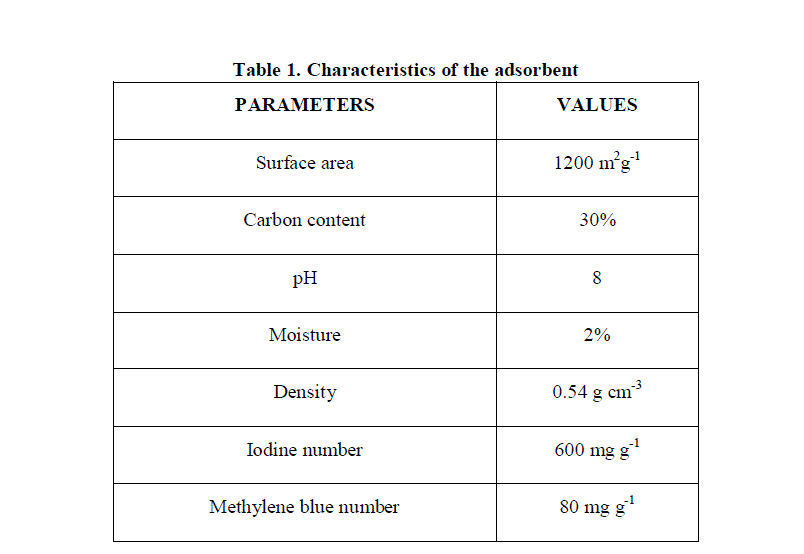
| The characterization of coconut shell activated carbon is presented in Table 1. As seen the carbon had high carbon content (30%), high surface area (1200 m2g-1), good number of micropores and macropores. |
 |
| 4.2 Effect of contact time |
| The percentage Dye sorbed were estimated at different times keeping the other parameter fixed and the results are shown in fig.1. It was observed that the percentage of removal of Rhodamine B increase with respect to contact times. 3hrs was taken as the equilibrium time for subsequent experiments. |
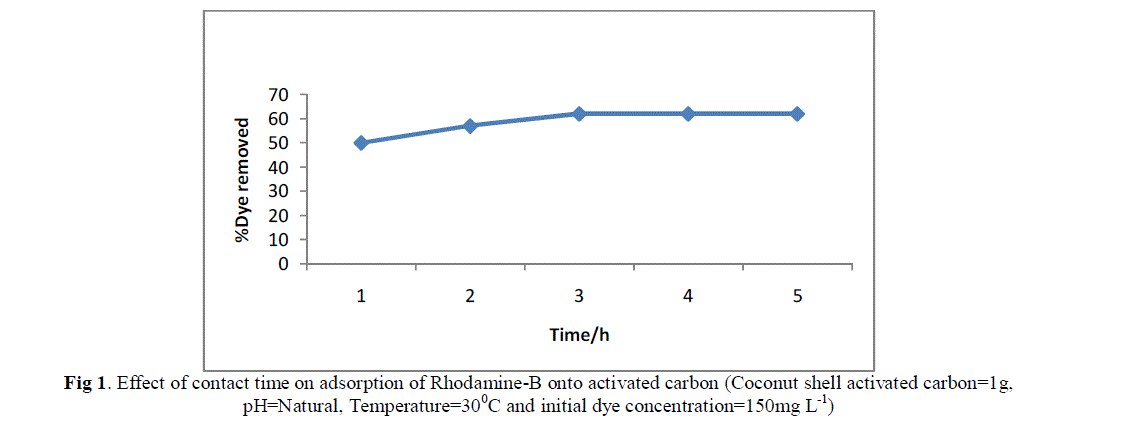 |
| Fig 1. Effect of contact time on adsorption of Rhodamine-B onto activated carbon (Coconut shell activated carbon=1g, pH=Natural, Temperature=300C and initial dye concentration=150mg L-1) |
| 4.3 Effect of pH |
| Because the initial pH of solution can significantly influence adsorption of dyes, the effects of pH on dye adsorption on the activated carbon was studied. The dye removal was maximum at acidic condition (< 7). The possible reason for such type of behaviour is due to the fact that Rhodamine B molecules are positively charged. As initial pH of the test solution increased, the number of negatively charged adsorbent sites increased and positively charged adsorbent sites decreased which favours the adsorption of positively charged dye cation due to electrostatic attraction. Thus at higher pH, adsorption will be more and at lower pH, adsorption will be lesser. |
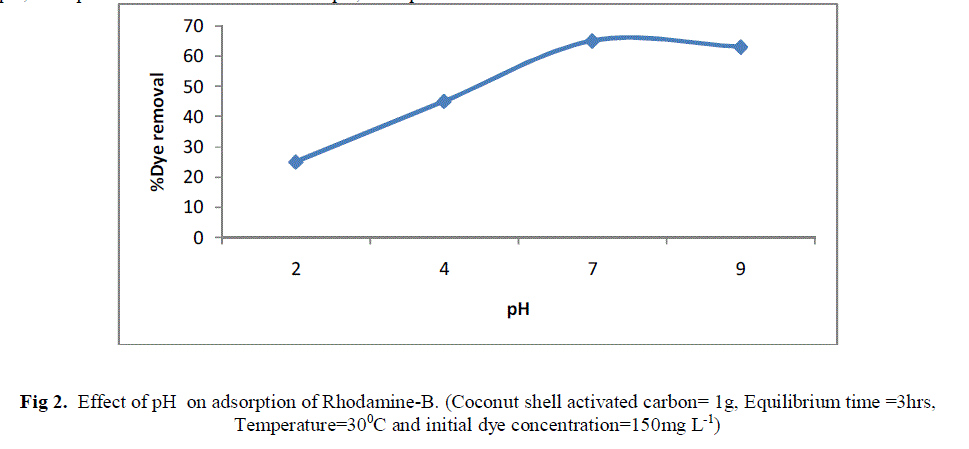 |
| Fig 2. Effect of pH on adsorption of Rhodamine-B. (Coconut shell activated carbon= 1g, Equilibrium time =3hrs, Temperature=300C and initial dye concentration=150mg L-1) |
| 4.4 Effect of Dye Concentration |
| The influence of dye concentration on percentage dye adsorbed was studied in the range of the effect of dye concentration was studied by keeping the adsorbent dose constant at 1g. The concentration of dye was in the range from 150-400 mg L-1. As shown in Fig 3. there was regular decrease in percentage of colour removal when the concentrations of the dye were increased. A decrease in colour removal upto 6% was observed when the concentration of RB was increased from 150-400 mg L-1 . However, it had been seen that the decrease in percentage colour removal was more pronounced at higher concentration. |
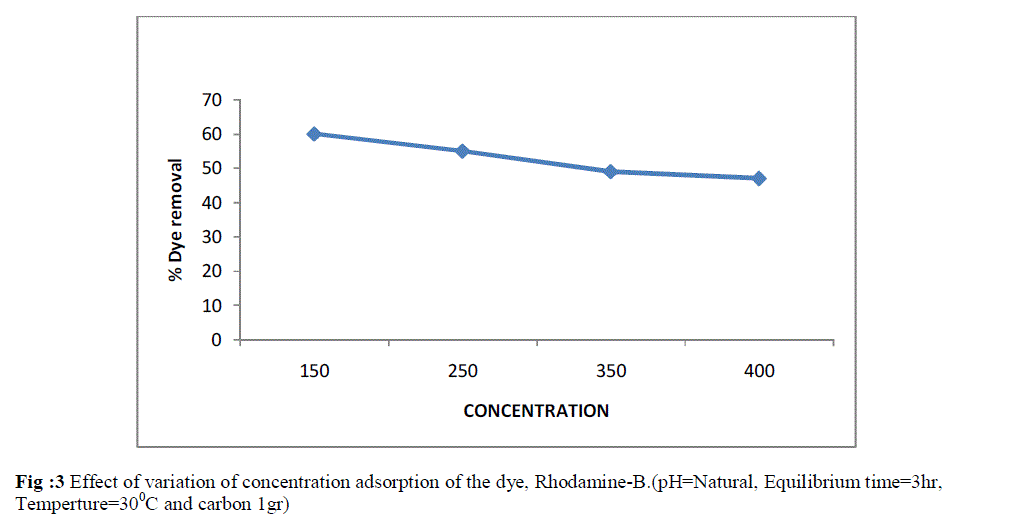 |
| Fig :3 Effect of variation of concentration adsorption of the dye, Rhodamine-B.(pH=Natural, Equilibrium time=3hr, Temperture=300C and carbon 1gr) |
| 4.5 Effect of sorbent dose |
| The effect of sorbent dose on the removal of the dye is shown in Fig 4. The percentage of the dye sorbed increased as the sorbent dose was increased over the range, 1-3 g. The adsorption of the dye increased from 60 to 70% with the increase in sorbent dose. This conveyed the idea that increase in dosage increase the availability of active sites to the dye molecules. |
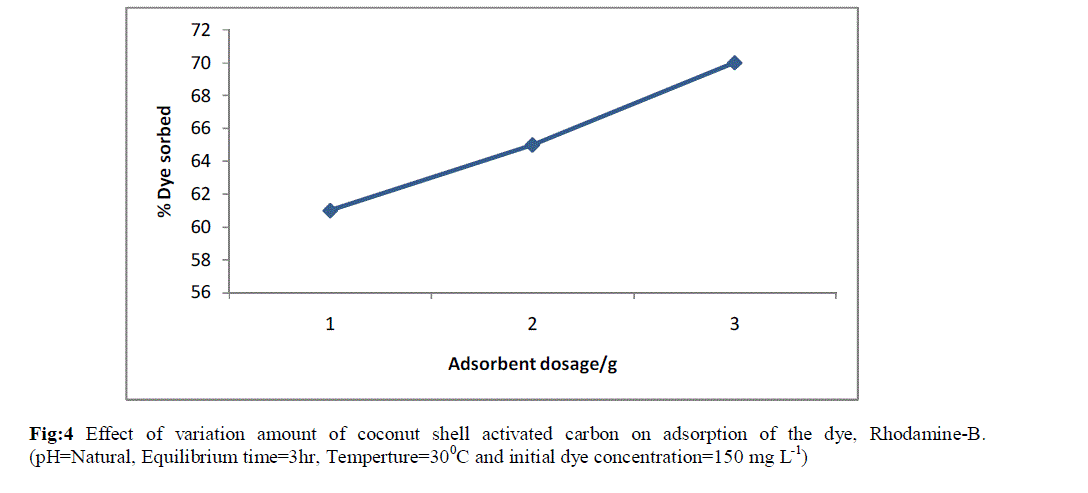 |
| Fig:4 Effect of variation amount of coconut shell activated carbon on adsorption of the dye, Rhodamine-B. (pH=Natural, Equilibrium time=3hr, Temperture=300C and initial dye concentration=150 mg L-1) |
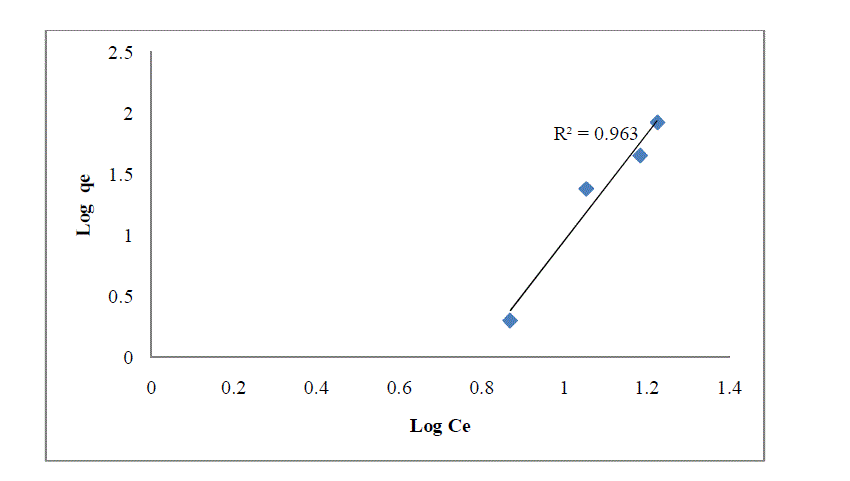
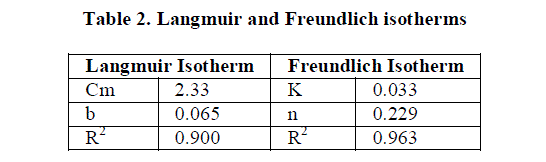
| 4.6 Freundlich adsorption isotherms |
| The data obtained from batch experiments were fitted to Freundlich and Langmuir adsorption isotherms. |
| The Freundlich isotherms is represented as |
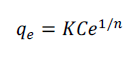 |
| The Linear form of Freundlich isotherm is represented as |
| Where is the amount of dye sorbed per unit weight of the adsorbent, is the equilibrium concentration (mg L-1), K and n are the empirical constants and their values were obtained from the intercepts (log k) and slopes(1/n) of the linear plot of log qe versus log Ce. |
 |
| 4.7 Langmuir Adsorption Isotherm |
| The Langmuir isotherm is represented as follows |
| Where C is the concentration of the dye solution at equilibrium, is the mass of the dye adsorbed per gram of the adsorbent. C is the mass of the dye that 1gm of adsorbent can adsorb when the monolayer is complete and b is the isotherm constant for particular adsorbate-adsorbent combination. The Cm and b values were calculated from the intercept (1/Cm) and slope (1/bCm) of linear plots of 1/qe versus 1/Ce. |
 |
CONCLUSION |
| This study proved that activated carbon produced from coconut shell were effective adsorbent for Rhodamine B dye. Removal of Rhodamine B will be better at acidic pH=7, moderate initial concentration (150 mg L-1) and high adsorbent(1 grm). |
References |
|