ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Talabi, A. O1*, Afolagboye L. O1, Aladejana, J. A2, Akinola, O. O1, Aturamu, A. O1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This study focused on assessment of possible impacts of artisanal and small scale mining activities on groundwater in Ijero-Ekiti. Thirty groundwater samples (2 from the mining pits, 5 in the vicinity of the mining pits and the remaining 23 randomly selected from other parts of the study area) were collected and analysed for the anions and cations using Ion Chromatography and Atomic Absorption Mass Spectrometer methods respectively. Physical parameters (Temp.°C, pH, ECμS/cm) were measured in-situ using a Multi-parameter TestrTm 35 series Meter. The average values of pH (8.36), EC (371.40μS/cm) and average ionic concentrations in mg/L {Ca2+ (18.21), Mg2+(7.45), Na+(43.10), K+(28.56), HCO3 -(20.15), SO4 2-(8.41), Cl-(256.34) and NO3 -(0.38)} for the samples (7) from the mining pits and their vicinity showed no significant difference from the average concentrations of groundwater samples from the remaining 23 samples randomly selected from other parts of the study area except that more solutes were introduced into the groundwater system as residence time and length of transportation increase. The 23 samples have average values of pH (8.41), EC (558.91μS/cm) and average ionic concentrations in mg/L {Ca2+ (53.94), Mg2+ (20.49), Na+(45.79), K+(64.98) HCO3 -(29.62), SO4 2-(13.82), Cl-(304.63) and NO3 -(0.38)}. Increase in solute input into the groundwater system as shown by the 23 samples from other parts of the study area might equally arise from lithological variations. The order of cations concentration in the study area is Ca2+ >Na > K+ > Mg2+ while that of anions is Cl-> HCO3 -> SO4 2-> NO3 -. All ions concentrations are within WHO standard for drinking water quality except chloride that exceeded it in few locations due to local anthropogenic contamination. The Piper trilinear diagram indicated Ca–(Mg)- Cl-(SO4) and Na-(K)-Cl-(SO4 as the dominant water. All water samples have low sodium hazard as well as low– medium salinity hazard while all irrigation indices (sodium absorption ratio, residual sodium bicarbonate and Kelly ratio) showed that the groundwater is suitable for irrigation. This study revealed that the groundwater of Ijero is low mineralized, chemically potable and suitable for irrigation while artisanal and small-scale mining activities have no significant impact on the groundwater.
Keywords |
| Artisanal, low mineralized, anthropogenic contamination, salinity hazard. |
INTRODUCTION |
| Artisanal Miners, in the absence of the required safe mining knowledge or any regulations or standards operate in difficult and often very hazardous conditions whereby toxic materials can be released into the environment, posing large health risks to the Miners, their families and surrounding communities. Some environmental hazards of mining activities are; mercury pollution, water pollutions, landscape degradation, destruction of habitats, loss of organic soil and deforestation. A special mention must be made of the environmental degradation caused by the illegal mining of gemstone. Because of the uncontrolled manner the illegal Miners operate, a lot of damage is done to the environment by haphazard pitting and trenching of the ground [1], [2] and [3]. |
| Ijero-Ekiti and environ has been considered a mineralized area in Ekiti State southwestern Nigeria. Records indicated that the area has diverse mineral deposits including metallic (cassiterite, tantalite, columbite), industrial (kaoline and marble), ceramic (clay and feldspar), speciality metal (lepidolite) and gem stone (aquamarine, smoky quartz and tourmaline), [4]. Ijero – Ekiti has estimated feldspar deposit of 3.76 million metric tons [5] apart from unestimated talc deposits in the area. Natural resources exploitation, exploration, mining and processing have caused different types of environmental damages which include ecological disturbances, destruction of natural flora and fauna, pollution of air, water and land, instability of soil and rock masses, landscape degradation, desertification and global warming. The environmental damage has in turn resulted in waste of arable land as well as economic crops and trees [6]. The current state of mining in Ijero area is being carried out by Artisanal, Small scale and Illegal miners whose operations are executed in haphazard manner without supporting infrastructure. Many of the Miners have low level of education and they do not know their environmental obligations under the Minerals and Mining Act, and that the adherence to best global practices in mining is a vital tool for the promotion of sustainable growth in the industry. In view of this development, little attention was paid to safety and environmental degradation. These actions have untold story on groundwater system as the debris arising from the illegal mining activities are washed off by surface run-off especially during rainy season. Invariably, some of the particulates end up in the groundwater aquifers of the area. In addition, the geological complexities of rock units (migmatite gneiss and metasediments of the Basement complex, pegmatite bodies associated with the schist belt and Basement gneiss intruded by pegmatites and quartz veins) in the study area are prone to differential weathering. The highlighted crystalline rocks formed by interlocking silicate minerals such as quartz, feldspars, micas, hornblende, pyroxenes, olivine and a host of minor accessories are exposed or made culpable to weathering through exploration and illegal mining of mineral deposits in the area. Chemical weathering involves the dissolution of these minerals resulting in the formation of both soluble as well as solid phase products. Some of the dissolved solids got infiltrated into the groundwater system of the study area resulting in modification of its composition. Although mining provides a variety of socio-economic benefits but its environmental costs, if not well handled can be massive in terms of land conversion and degradation, habitat alteration, water and air pollution [3]. Hence, this study assesses only the impact of mineral exploration and exploitation on the groundwater system of the study area paying attention to groundwater potability and irrigation quality assessments. |
LOCATION AND GEOLOGY |
| Ijero area comprises of Ijero-Ekiti with other component towns including Ikoro- Ekiti, Iroko- Ekiti, Ayegunle-Ekiti, Ipoti-Ekiti, Iloro-Ekiti, Temidire-Ekiti, Ilukuno-Ekiti and others are Epe-Ekiti, Ara-Ekiti, Araromi-Ekiti, Oke Oro-Ekiti and Odo Owa -Ekiti. The area covers 473.5km2. This study is limited to Ijero-Ekiti where most of the mineral exploration and exploitation activities were carried out. The study area falls within the Basement Complex of Southwestern Nigeria. It lies within Latitudes7° 48' to7° 49.5' North and Longitudes 5° 3' to 5° 4' East (Fig. 1). The topography is rugged characterized with low lying hills that are generally 250m above sea level. Geologically, the study area is underlain by crystalline rocks of Precambrian Basement Complex of southwestern Nigeria. The crystalline rocks have been categorized into the migmatite gneiss complex, the schist belts and the Pan African granites [7]. The crystalline rocks have suffered polycyclic deformations which were as a result of several techtenothermal events including the Liberian, Eburnean, Kiberian and Pan African orogenies. The Pan African orogeny witnessed the emplacement of the older granites and affected the pre-existing meta-igneous and metasedimentary rocks. The lithologic assemblages include migmatite gneisses, schists, quartzite and pegmatites all of which are Precambrian – Cambrian age. [4] indicated in his paper delivered at Ekiti State Economic Development Summit that minerals occurred in specific geologic rocks in the study area. Marble, an industrial mineral occurs in the migmatitic gneisses and metasediments while cassiterite, columbite, lepidolite and tantalite (metallic minerals) occur in pegmatite bodies associated with schist and amphibolite schists. Other mineral components in varied rock units of the study area include kaolin formed from gneiss intruded by pegmatite and quartz veins, feldspars associated with pegmatite bodies as well as disseminated gemstone (tourmaline, aquamarine and smoky quartz) in pegmatite. |
METHODOLOGY |
| The field work activities of this study were accomplished in January, 2014 during which 30 groundwater samples (2samples from the mining pit, 5samples close to the mining pit and 23 samples spreading over the remaining area) were collected for chemical analysis while in-situ parameters (temperature, pH and EC) due to their unstable nature to avoid unpredictable changes in characteristics as per the standard procedures [8] were measured on the field at each location employing a Multi-parameter TestrTm 35 series Meter which was calibrated before and during fieldwork using buffer solutions as prescribed by the manufacturers. 12 Channel GPS etrex Garmin was used at each location to record the coordinates and altitudes to produce the sample location map (Fig. 1). At each sampling location, groundwater sample (meant for ion analyses were filtered through 0.45 μm membrane filter [9] to allow the removal of particles and bacteria that could modify its content) was collected into a plastic collector after thorough rinsing with distilled water and the water to be sampled. |
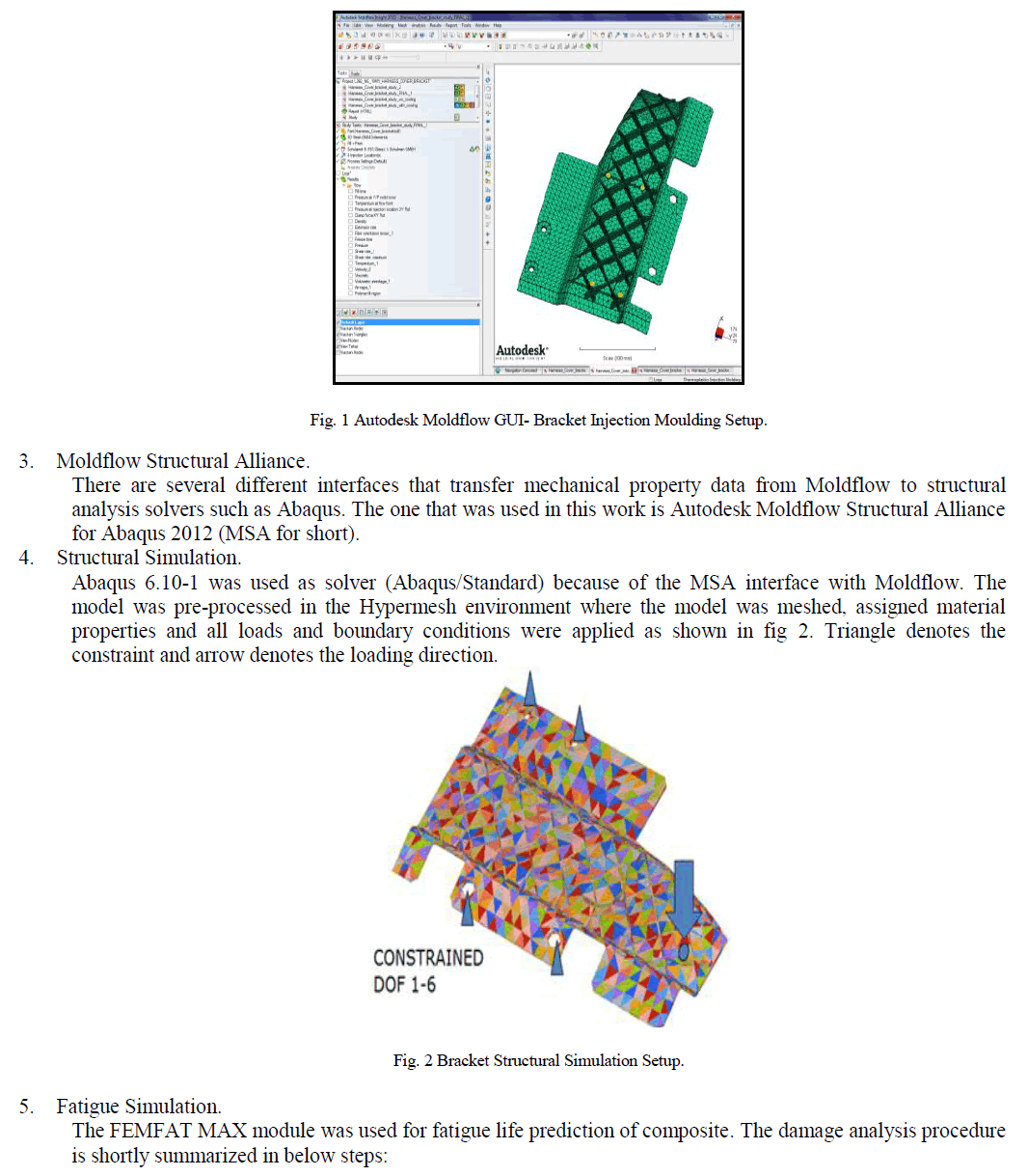 |
| The samples for cation analysis were acidified to a pH<2 by adding 1% nitric acid [11]. The samples were preserved airtight in order to minimize oxygen contamination and the escape of dissolved gases. The samples were stored in icechest containers at about 4°C prior to laboratory analysis to exclude microbial activity and unwanted chemical activity. The samples were transported to the Soil and Water Laboratory of Fatlab Nigeria Company Agbowo UI, Ibadan for ions analysis employing the Flame Technique in a High Resolution Continuum Source AAS (ContrAA700) for cations and Ion Chromatography (Dionex ICS-900) for anions determinations. |
DATA EVALUATION |
| Data obtained from the analyses were subjected to statistical evaluation employing SPSS 17. Other graphical plots employed in this study include Piper tri-linear diagram [12], Schoeller diagram [13], Gibb’s plots [14] and [15] plot. Rockwork software was employed for the plot of the Piper and Schoeller diagrams while Gibb’s and Chadha diagrams were plotted employing Microsoft excel. Irrigation quality parameters including sodium adsorption ratio, residual sodium bicarbonate and magnesium absorption ratio were estimated employing the following under listed equations: The Sodium Adsorption Ratio (SAR) was calculated by the following equation given by [16] as: |
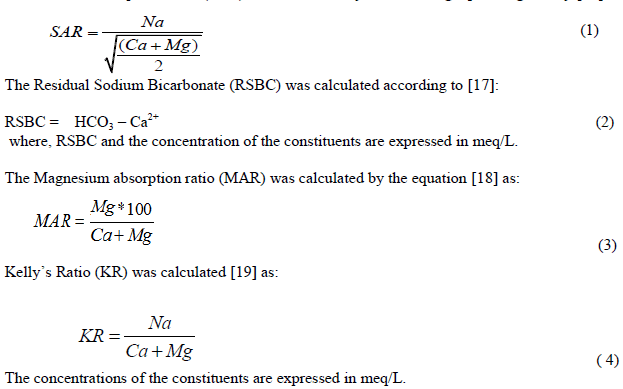 |
RESULTS AND DISCUSSIONS |
HYDROGEOCHEMISTRY |
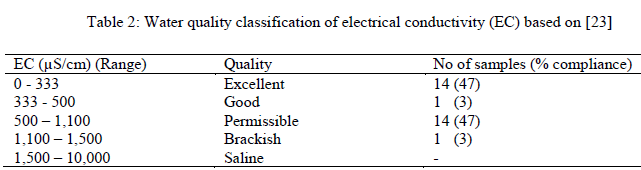
| The overall statistical summary of results of measured parameters of groundwater in the study area along with [20] approved standard for drinking water quality is presented in Table 1. Generally, pH of groundwater in the study area ranged from 7.40 – 9.50 (av. 8.42), indicating alkaline water. However, 50% of the groundwater has pH (pH > 8.5) values outside the range of approved WHO standard for drinking water (pH > 8.50). Corrosion effects may become significant below pH 6.5 and the frequency of incrustation and scaling problems may be increased above pH 8.5. Insofar as pH affects the unit processes in water treatment that contribute to the removal of viruses, bacteria and other harmful organisms, it could be argued that pH has an indirect effect on health. The acceptable range for drinking water pH is herefore from 6.5 to 8.5. In general, waters with a pH within this range can be stabilized with respect to corrosion and incrustation by simple pH adjustment. By keeping the pH below 8.5, the rate of chlorine disinfection is increased and the production of trihalomethanes is reduced [21]. The results further revealed low solute input into the groundwater system as exemplified by EC that ranged from 42.00 – 1187.00 (av. 536.73) μS/cm. The wide variations in EC were expected in view of the terrain of study with varied elevation (49.24 – 562.10m) which signified rugged topography where bedrocks response to differential weathering and other geologic agents also varied exceedingly. Thus, movement and distribution of ionic constituents in the groundwater was guided by the nature of the terrain. EC and TDS are related and in this study, all EC and TDS values fell within [20] approved standard for drinking water. Total dissolved solid represents dissolved minerals present in the groundwater of the area under investigation. TDS and EC are important in quality assessment of water due to their effect on the corrosivity of a water sample and their effect on the solubility of slightly soluble compounds such as CaCO3 [22]. Electrical conductivity is an indirect measure of salinity in many areas, which generally affects the taste and thus has significance on the user acceptance of the water as potable [23]. Majority of the groundwater samples (97%) are within the permissible limit of water quality classification based on electrical conductivity (EC) (Table2). The remaining one sample (3%) with EC>1,100μS/cm might be as a result of local anthropogenic contamination of groundwater in that location. TDS assessment of water quality according to [24], indicates that TDS values between 0 and 1000mg/L signifies freshwater while those from 1000 -10,000mg/L are brackish water. Twenty seven (90%) of the groundwater samples in this study fell in the freshwater category while the remaining three (10%) samples were brackish water consequent of anthropogenic activities. This observation indicates that the groundwater is low mineralized and represents recent water that has low residence time and low water- rock interactions [25]. The Ca2+ ranged between 0.54 and 139.21 (av. 49.16) mg/l while Mg2+ ranged from 0.15 - 59.52 (av. 18.40) mg/L. The alkaline (sodium and potassium) ranged from 8.42 – 112.45 (av. 46.55) mg/L and 1.21 – 198.57(av. 57.47) mg/L respectively. Major anions revealed HCO3 - ranging from 6.10 – 51.85 (av. 28.31) mg/L, SO2- 4 ; 0.86 – 42.67 (av.12.69) mg/L, Cl-; 57.68 – 792.03 (av. 308.19) mg/L and NO3 -; 0 – 1.15 (av. 0.37) mg/L |
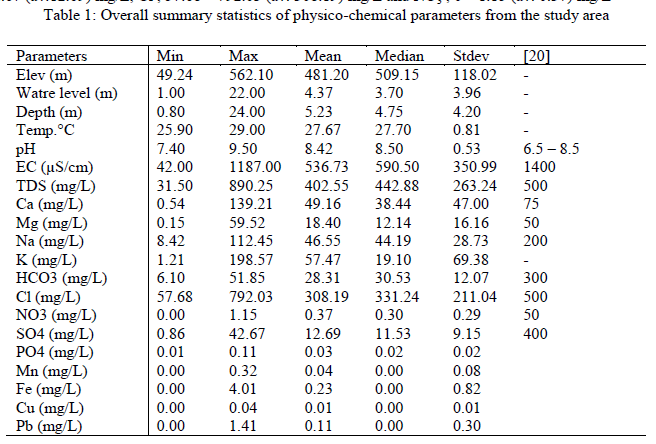 |
| The order of cations concentration in the study area is K+ > Ca2+ >Na > Mg2+ while that of anions is Cl-> HCO3 -> SO4 2-> NO3 -. All ions concentrations are within [20] standard for drinking water quality except chloride that has 8 samples (26.67%) with values above recommended WHO standard probably arising from local anthropogenic contamination of the groundwater samples. Low trace elements (Fe (av.0.23) mg/L, Mn (av. 0.04) mg/L, Cu (av. 0.01) mg/L concentrations were observed in this study except for Pb (av. 0.11) mg/L) that exceeded approved WHO standard of 0.01mg/L in five locations representing 16% coverage of the area. In addition, low concentrations of NO3 - were also recorded signifying low level of anthropogenic contamination of groundwater in the area. The alkaline ions (Na+ and K+), chloride (Cl-) and impurities got introduced into our environment through runoff from rain, splash and spray from vehicle and wind. Invariably, the ions and impurities could be transported onto vegetation and into the soil, groundwater, storm drains as well as surface water and may impact negatively on the environment. |
 |
| Chloride is completely soluble, very mobile, toxic to aquatic life and has significant impacts on vegetation and wildlife. Up till this period of study, there is hardly any natural process by which chlorides are broken down, metabolized, taken up or removed from the environment. This probably explains the relatively high chloride concentrations in the groundwater of the study area. Sodium participates in ion exchange process and as such its transportation in the environment is not as prominent as chloride. The ion exchange process can result into alteration of the soil chemistry by replacing and releasing nutrients into the groundwater and surface water modifying soil structures and impacting the aquatic environment. Though, sodium concentrations in the groundwater of the study area fell within WHO approved standard for drinking water, excess of it, impacts on human health especially for individuals restricted to low sodium diets due to hypertension. The groundwater of the study area showed varied degrees of hardness. Water hardness is as a result of presence of cations (Ca and Mg) and anions (CO3, HCO3, Cl and SO4) in water. Employing the classification of [26] in which hardness of 0 – 75 meq/L is in the soft category, 75 – 150 in moderate hard, 150 – 300 in hard and > 300 in very hard category, 13 samples fall under soft hardness class, 5samples in the hard and 12samples in the very hard class. Water hardness has no known adverse effects; however, some evidence indicates its role in heart disease [27]. |
IMPACTS OF ARTISANAL AND SMALL SCALE MINING ACTIVITIES ON THE GROUNDWATER |
| Table 3 represents comparative summary statistics of physico-chemical parameters from mining pits/vicinity with those from other parts of the study area. The average values of pH (8.36), EC (371.40μS/cm) and average ionic concentrations in mg/L {Ca2+ (18.21), Mg2+(7.45), Na+(43.10), K+(28.56), HCO3 -(20.15), SO4 2-(8.41), Cl-(256.34) and NO3 -(0.38)} for the samples (7) from the mining pits and their vicinity showed no significant difference from the average concentrations of groundwater samples from the remaining 23 samples randomly selected from other parts of the study area except that more solutes were introduced into the groundwater system as residence time and length of transportation increase. The 23 samples have average values of pH (8.41), EC (558.91μS/cm) and average ionic concentrations in mg/L {Ca2+ (53.94), Mg2+ (20.49), Na+(45.79), K+(64.98) HCO3 -(29.62), SO4 2-(13.82), Cl-(304.63) and NO3 -(0.38)}. Increase in solute input into the groundwater system as shown by the 23 samples from other parts of the study area might equally arise from lithological variations. The bedrocks of the mining pits/vicinity is majorly calcgneiss into which pegmatite intruded whereas the other parts of the study area from which 23 samples were picked randomly have bedrocks comprising of pegmatite, biotite schist, calc-gneiss and quartzite. Response of these bedrocks to differential weathering differ s and as such differences in solute input into the groundwater. Artisanal and small scale mining activities in the study area have not impacted the groundwater of the study area negatively. |
 |
CORRELATION |
| Correlation is a statistical technique that can show whether and how strongly pairs of variables are related. Pearson’s correlation provided the direction and the strength of linear relationship between any two variables. Data from this study were subjected to Pearson’s correlation evaluation and the result of the correlation is presented in Table 4. The result revealed that EC has positive correlation (r>0.78) with the major cations but low positive correlation (0.163 r 0.366) with the major anions. In addition, the cations have positive correlations (r>0.533) among themselves while the anions have low positive correlations except for Cl and SO4 (r = 0.61), Cl and NO3 (r = 0.526) and SO4 and NO3(r = 0.729). These trends of correlations indicate that Cl, SO4 and NO3 are from the same sources, mostly anthropogenic sources while geogenic sources arising from minerals dissolution/weathering were more significant with respect to the cations. However, HCO3 shows relatively high positive correlation with Ca, Na and K (0.310 r 0.475) signifying that some of the HCO3 concentrations in the groundwater of the study area were consequent of input of solute into the water from minerals dissolution/weathering. Further, The source of the dissolved ions in groundwater can be understood by Gibbs diagram [14]. The Gibbs diagram is useful in hydrogeochemical evolution of groundwater. The mechanism controlling groundwater geochemistry (reaction between groundwater and aquifer minerals) which has a significant role in water quality and in understanding of the genesis of water can be evaluated [14]. Evaluation of the sources of various ions in the groundwater of the study area was carried out employing a plot of the logTDS against Na+ +K+/(Na+ + Ca2+) and Cl/Cl+HCO3 ratio (Fig. 2). The diagrams revealed that the groundwater has modified its chemistry from the weathered materials derived from the underlying bedrocks. However, correlations matrix revealed that Cl, SO4 and NO3 originated from similar sources. Nitrate (NO3) has been known to originate from anthropogenic activities and in accordance with the work of [28], Cl, SO4 and NO3 input into the groundwater of the study area were from surface phenomena arising from anthropogenic activities in the environment. |
 |
HYDROGEOCHEMICAL FACIES |
| Determination of the origin of the chemical composition of groundwater as well as the relationship between water and rock chemistry particularly as they relate to groundwater movement constitute major domain in hydrogeochemistry. In this regards [12] diagram has been the basis for several important interpretations of the hydrogeochemical data. Major ions of water samples are plotted as percentages of mill equivalents in two base triangles setting the total cations and the total anions at 100%. The data points in the two triangles are projected onto an adjacent grid (diamond shape) representing the composition of the water. This plot reveals useful properties and relationships for large sample groups with clustering of data points to indicate samples that have similar compositions. Most natural waters contain relatively few dissolved constituents, with cations (metals or bases) and anions (acid radicals) in chemical equilibrium with one another. Modification of this initial chemical composition begins as soon as the waters percolate into the subsurface of the earth. To assess the evolution of hydrochemical parameters of groundwater in this study, major cations and anions were plotted on the Schoeller’s and Piper’s diagrams (Figs.3 and 4 respectively). The Schoeller diagram represents major ion analyses in meq/l to demonstrate different hydrochemical water types on the same diagram. This type of graphical representation has the advantage that, unlike the trilinear diagrams, actual sample concentrations is displayed and compared. In this study, the Schoeller diagram revealed the dominance of Ca and Na cations as well as Cl anions in the groundwater of the area (Fig. 3). |
| Chemical data of groundwater samples from the study area on a Piper-tri-linear diagram (fig.4) reveals the analogies, dissimilarities and different types of waters in the study area as Na-K-Cl-SO4 water type (46.67%), Ca-Mg-Cl-SO4 water (33.33%) and mixed water type (20%). [15] has proposed a simpler new diagram that was modified from Piper diagram for geochemical data presentations. |
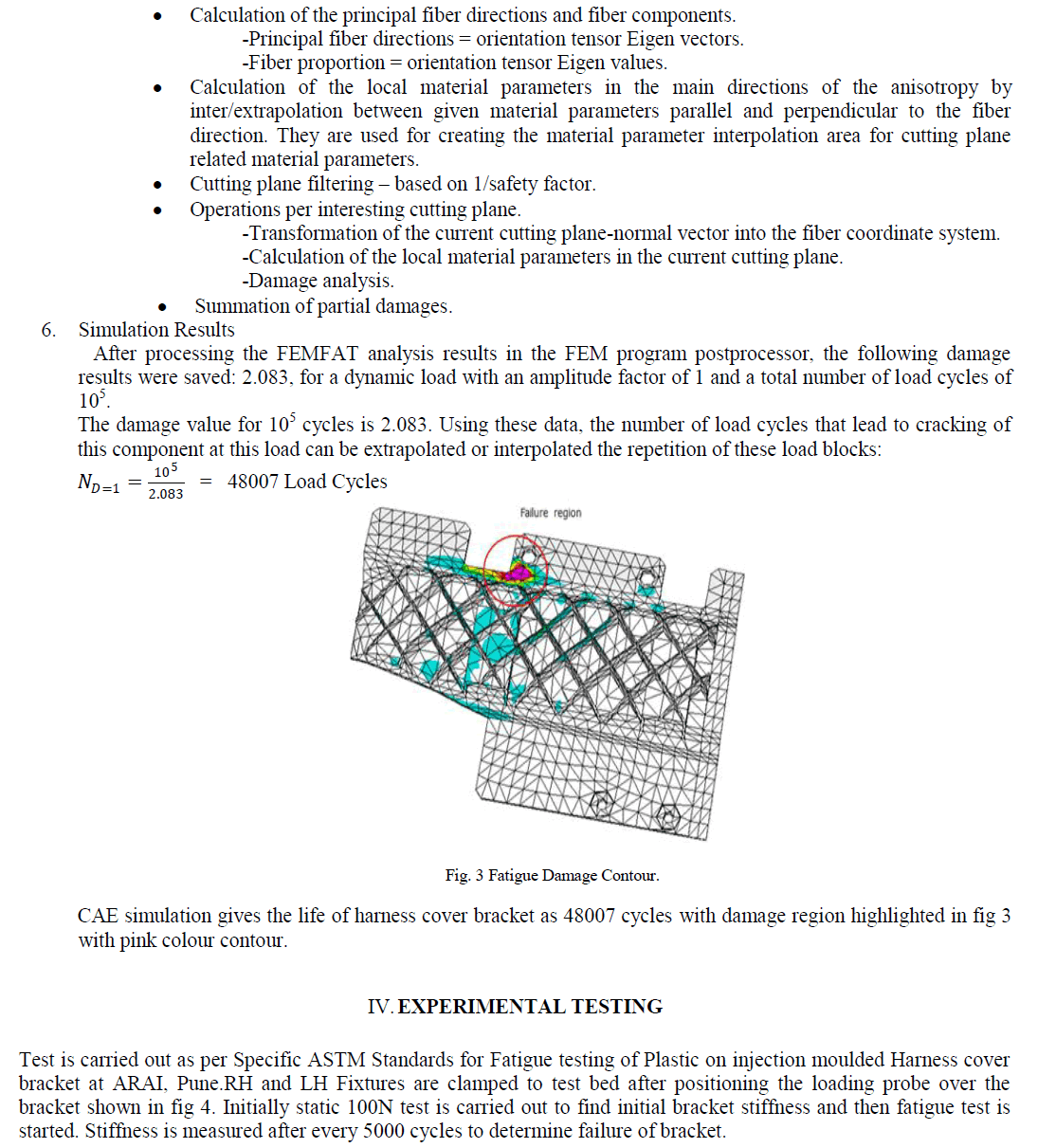 |
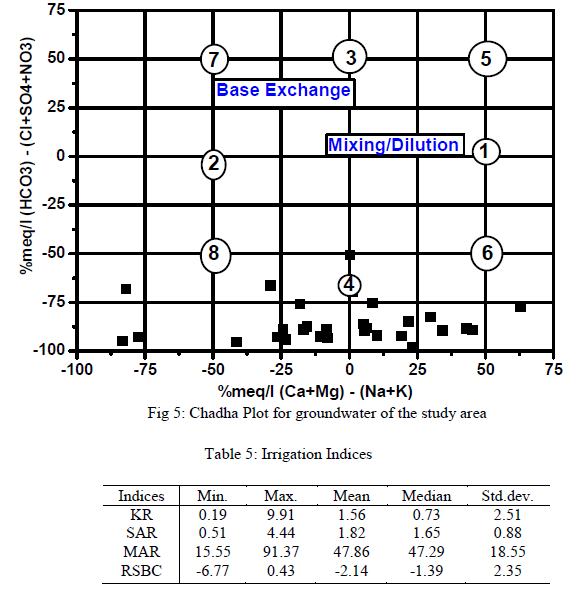
| Results of analyses from the study area were plotted on the proposed diagram to test its applicability for geochemical classification of groundwater and to study hydrochemical processes (Fig.5). The plot shows that 14 of the groundwater samples fall under the subdivision of alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions (Ca-Mg- Cl-SO4 water type) while 16 samples were in the reverse order representing Na-K-Cl-SO4 water type. Both Piper diagram and Chadha support the dominance of Na-K-Cl-SO4 water type in the study area. |
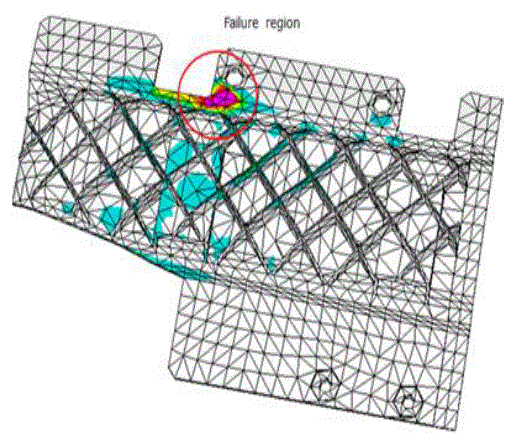 |
IRRIGATION QUALITY ASSESSMENT |
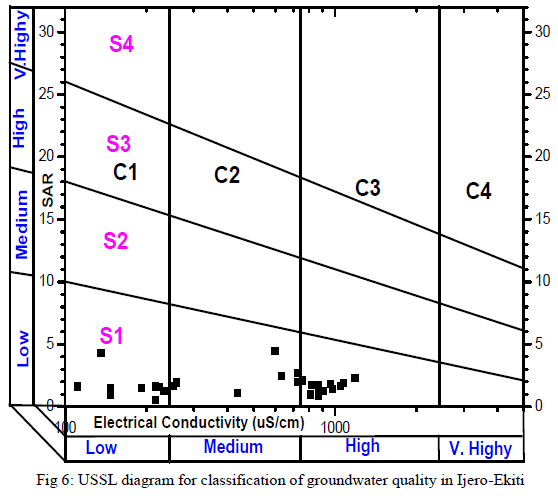
| Groundwater in the study area was assessed for irrigation by consideration of acceptable range of: Electrical Conductivity (μS/cm), Sodium Absorption Ratio, Kelly’s Ratio, Residual Sodium Bicarbonate and the Magnesium Adsorption Ratio. Groundwater always contains dissolved substances referred to as salts. The salts present in the water affect the growth of plants directly as well as the soil structure, permeability and aeration which indirectly affect the plants growth. The total concentration of soluble salts in irrigation water can thus be expressed for the purpose of irrigation water assessment employing the classification of [29] in which water with EC<250μS/cm are in the low salinity class, 250 - 750μS/cm in the medium salinity, 750 - 2250μS/cm in the high salinity and 2250 – 5000μS/cm in the very high salinity. In this study 60% of the groundwater samples were in the low – medium salinity and the rest 40% in the high salinity. The summary of the remaining irrigation indices for rating irrigation water quality are presented in Table 5. SAR ranged from 0.51 to 4.44. SAR represents the intake of Na+ by soil. It is the proportion of sodium to calcium and magnesium which affect the availability of water to the crop. All SAR values were less than 10 and the groundwater is considered as good irrigation water suitable on all agricultural soils [30]. The plotting of SAR values in USSL diagram (Fig. 5) indicates that all the samples have low SAR value. Out of 30 samples 10 samples in C1-S1 field, 9 in C2 –S1 and 11 in C3 –S1, implied that no alkali hazard was anticipated to crops in the study area. Similarly, KR ranged from 0.19 – 9.90, with 19 samples (63% of total samples) having KR<1indicating that majority of the groundwater samples were suitable for irrigation [31].High magnesium to calcium in groundwater will increase its salinity and adversely affect crop yields [32]. In this study, MAR ranged between 15.55 – 91.37 (av.47.86). Nineteen out of the 30 groundwater samples fell within the permissible limit of 50 representing 63% compliance [33]. The suitability of water for irrigation purpose is influenced by the bicarbonate and carbonate concentrations. The water with high RSBC has high pH. Therefore, land irrigated with such water becomes infertile owing to deposition of sodium carbonate [34]. The residual sodium bicarbonate values of groundwater samples from the study area vary from -6.77 – 0.43 meq/L. The RSBC values in this study are less than 1.25 meq/L and are therefore considered excellent for irrigation purposes [35]. |
 |
 |
CONCLUSIONS |
| Interpretation of hydrochemical analysis in this study revealed that the groundwater in study area is low mineralized with majority of groundwater samples in fresh hardness class (43.33%), hard ((16.67%) and very hard (40%) class. The sequence of the abundance of the major ions is in the following order: K+ > Ca2+ >Na > Mg2+ and Cl-> HCO3 -> SO4 2-> NO3 -. The alkalis are more than alkali earths metals with strong acids dominating the weak acids. Low trace elements and NO3 - concentrations signified low level of anthropogenic contamination of the groundwater. Virtually, all water samples fell in the rock dominance area in Gibbs plot indicated that the groundwater has modified its chemistry from the weathered materials derived from the underlying bedrocks. Furthermore, the dominant hydrochemical facies of the groundwater were Ca-Mg- Cl-SO4 and Na-K-Cl-SO4. |
| Irrigation quality assessement employing SAR, KR and RSBC indices revealed that the groundwater in the study area is good and suitable for irrigation on all agricultural soils. However, assessment of irrigation quality employing MAR indicated that only 63% of the groundwater samples were suitable for irrigation. No significant difference in ionic concentrations of groundwater samples from the mining pits/vicinity and samples from the other parts of the studyv area. This study has revealed that the artisanal and small-scale mining activities in Ijero-Ekiti have not influenced the chemistry of groundwater in the area. The groundwater in the area was low mineralized, chemically potable and good for irrigation purpose. |
 |
References |
|