ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Inbasekaran1, R. Senthil2, G. Ramamurthy3, T.P. Sastry*4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A novel technique for glucose sensing using collagen based thin film and zinc oxide (ZnO) nanoparticles, the Zinc Oxide Collagen Glucose Sensor (ZnOCGS) presented here. The Zinc oxide is synthesized using the two medium namely treating with 1.Ethanediol medium (Ethylene glycol) and 2.Water medium. It is characterized using the analytical techniques such as Fourier Transform infrared spectroscopy (FTIR) and Fourier Transform-Raman spectroscopy (FT-Raman). The FTIR spectra of the materials obtained from the synthesis of water medium shows the zinc oxide nanoparticles absorption band at 437cm-1. The spectrum obtained from Ethanediol (Ethylene glycol) also shows the same absorption peaks as in the water medium. The results obtained using FT-Raman spectroscopy also indicates the same absorption peaks in both the mediums.
`
Keywords |
| Glucose Biosensor, Zinc oxide nanoparticles, collagen film, cyclic volt-meter. |
INTRODUCTION |
| Biosensors are analytical devices that comprise a biological recognition element and a suitable transducer, which are usually coupled to an appropriate data processing system (Fig.1.). The biological recognition element may be an enzyme, micro-organism, tissue or bioligand such as antibodies and nucleic acids. The transducer converts the physicochemical change due to the interaction of molecules with the receptor into an output signal. Optical, electrochemical, calorimetric, and magnetic, micromechanical and piezoelectric transducers can be employed in biosensors. Recognition elements can be immobilised on sensor support or sensor surface using different methods such as; entrapment, encapsulation, adsorption, capture and covalent attachment. A biochemical sensor is a small device consisting of a transducer covered by a biological recognition layer which interacts with the target analyte. The chemical changes resulting from this interaction are converted by the transducer into electrical signals. Electrochemical biosensors combine the analytical power of electrochemical techniques with the specificity of biological recognition processes to produce an electrical signal that is related to the concentration of an analyte [1]. In electrochemical biosensors, the transducer is an electrode. Based on the nature of the biological recognition process, two general categories of electrochemical biosensors can be defined: bio catalytic devices (utilizing enzymes, cells or tissues as immobilized bio components) and affinity sensors (based on antibodies, membrane receptors or nucleic acids)[2]. Electrochemical biosensors can be further divided into the sub-categories of potentiometric, amperometric and impedimetric biosensors depending on their mode of operation. Electrochemical biosensors are widely used in the medical field [3]. One of the most important applications of such devices is for the diagnosis and management of diabetes, a topic which has received a great deal of interest due to its urgent need and as a model system for sensor development. |
| Biosensors can be classified according to the recognition element as bio affinity sensors, enzyme sensors, transmembrane sensors and whole cell biosensors; or according to the transducer as electrochemical, optical, piezoelectric and calorimetric sensors. Bioaffinity sensors provide real time measurements to detect binding of bio molecules to each other. The antibody antigen (immunosensor), receptor ligand, DNA and RNA to nucleic acid / protein binding interactions can be detected by means of bio affinity sensors. While preferred methods of detection for catalytic sensors are usually electrochemical, the main methods of detection for bio affinity sensors are generally optical or piezoelectric. |
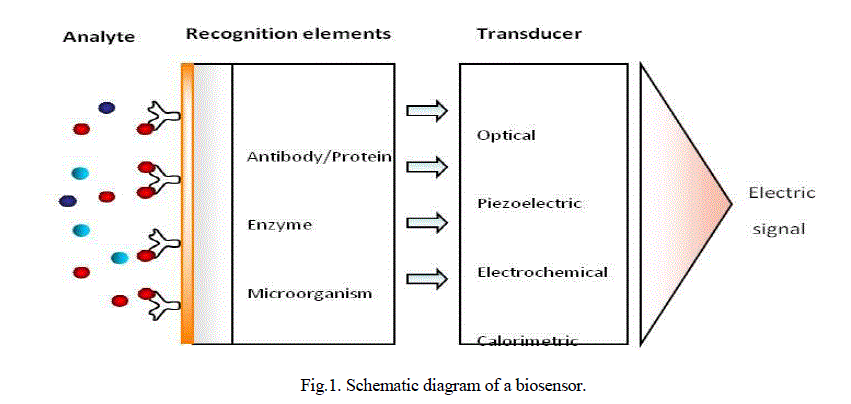 |
| Development of a bio sensing platform requires work related with biotechnology, molecular biology, material science, micro fluidics, that are summarised in Fig.2. Although, the commercialization of biosensor technology has significantly lagged behind the research output till now, development of micro and nanofabrication technologies for electronics and micro fluidics applications, in addition, use of nanotechnology in biological assay development, will pave the way towards next generation biosensors. As a result, it is essential to explore further research in next generation biosensors with possible commercialisation in mind. Concentrations below 3.9mM (70mg/dl) or above than 10mM (180mg/dl) are considered to be hypoglycaemic or hyperglycaemic, The middle layer using a spacer to test the glucose content in the sample used was glucose Oxidase. Using a standard multimeter, the peak voltage after 10 seconds was recorded 280mv for hypoglycaemia and 860mv for hyperglycaemia. |
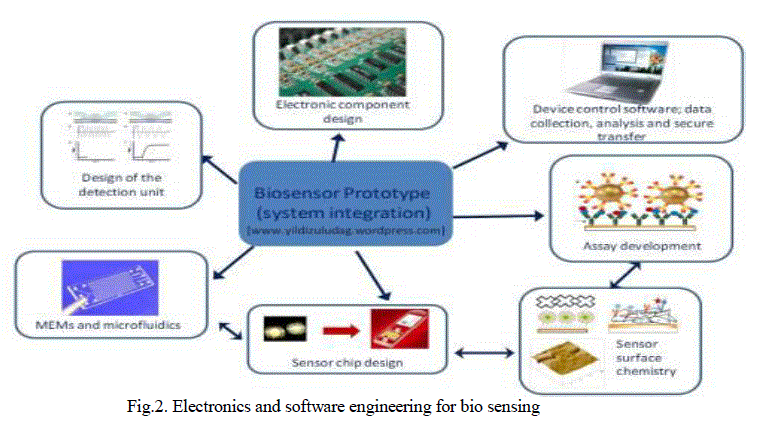 |
II. MATERIALS AND METHODS |
| 5.5 gm of Zinc Chloride is dissolved in 100ml of distilled water and heated up to 90°C. On the magnetic stirrer is placed a beaker containing zinc chloride. 16% sodium hydroxide solution is added to the zinc solution and stirred for 2 hours. The supernatant is solution removed using the pipette while the colloidal solution (bottom layer) is washed 5 times with distilled water. The washed sample is taken and dried at a temperature of 100°C for 30 minutes, this yields a powder of ZnO Nanoparticles. Preparation of Glucose Oxidase Enzyme solution: Sodium acetate solution (2.4mg/ml) is prepared and added with glucose oxidase (2mg/ml), mixed and pH is adjusted to 5.1. |
| Preparation of Zinc oxide nanoparticles collagen thin film glucose biosensors: One ml of ethylene glycol is added to100 ml of collagen solution (%), mixed well and 0.5g of ZnO is added to this mixture and mixed thoroughly using sonicator (10 W, 15 minutes), then this solution is used to dip the two different electrodes (copper and tin) to make current sensing element of the bio sensor. Fig.3. Show the Glucose Biosensors architecture. Zinc oxide nanoparticle based collagen impregnated with copper and tin electrodes of equal size (5cmx1cm) in three layers bonded together. We prepared two solution of glucose at 2.8mM and 16.7mM. |
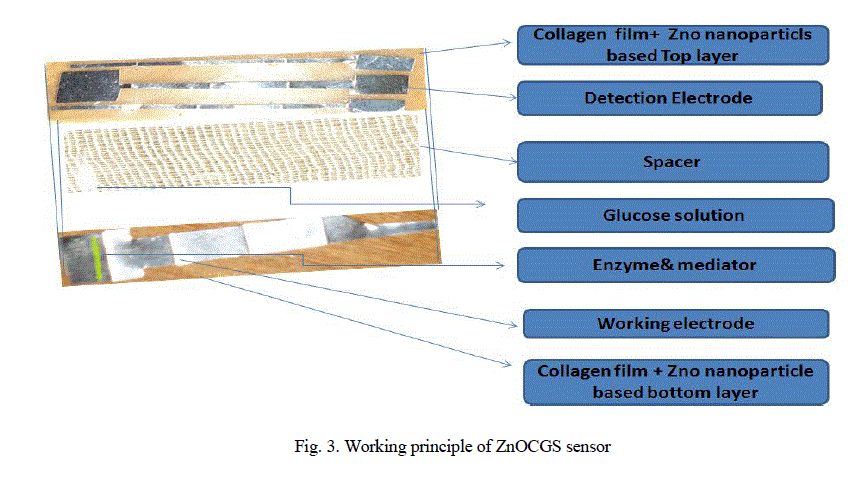 |
III. RESULTS AND DISCUSSION |
| In the Fig.4. Exhibits FTIR spectra for the sample as indicated in Fig.4., the broad absorptions at about 3430 and 1634 cm-1 are assigned to the hydroxyl groups of chemisorbed and/or physisorbed H2O molecules on the ZnO surface. A strong absorption |
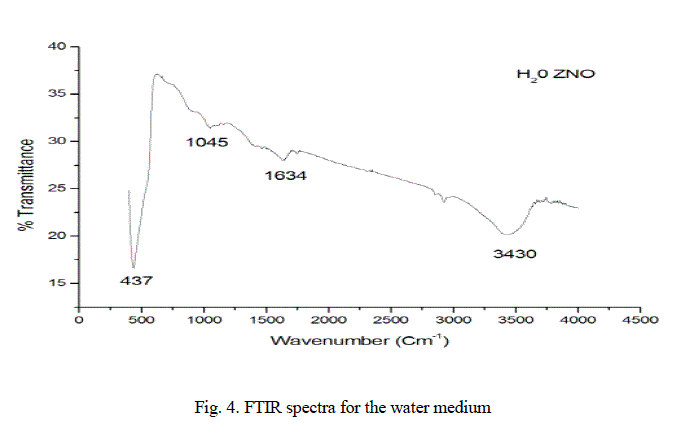 |
| band revealing the Vibrational properties of ZnO nanocrystals is observed for sample in the range of 650-450 cm-1; however, the absorption band of ZnO nanoparticles for our sample occurs at 437cm-1(Zheng ea al, 2007). |
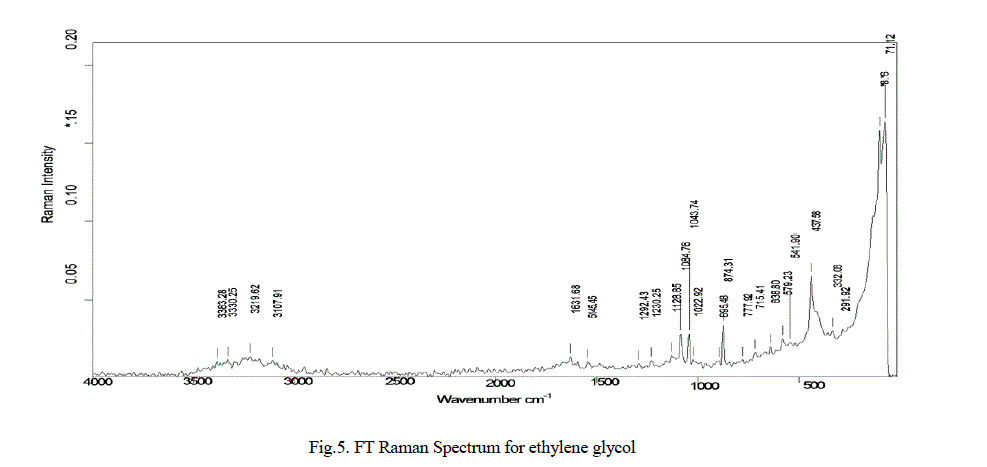 |
| In the Fig.5. Exhibits FT Raman spectra for the sample as indicated in Fig.5, the absorptions between at 437cm-1, 638 cm-1, 895 cm-1, 1084 cm-1, 1128 cm-1, 5145 cm-1, 1631 cm-1, are represents ZnO nanoparticle, Collagen and its amide absorptions. |
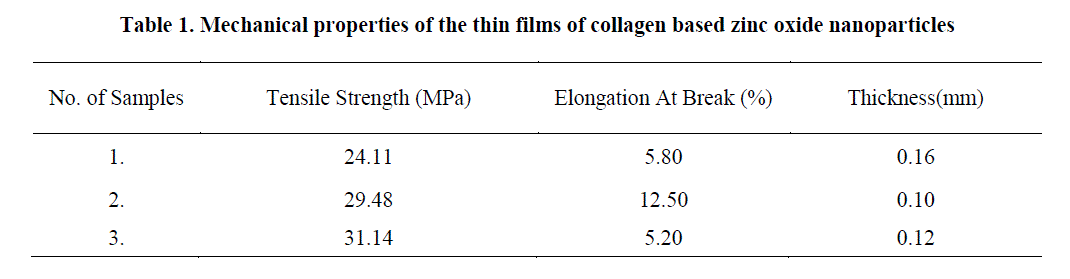 |
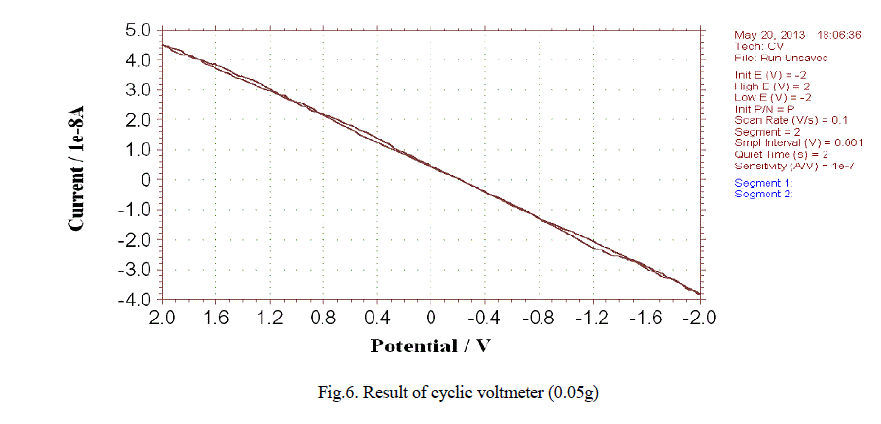
| In our study the material exhibits the desired thickness and good tensile strength as inferred from Table 1 Cyclic voltmeter test: Due to repeated occurrence of the peak at the desired wavelengths for both the medium the study further reveals an increase in current as the concentration of zinc oxide nanoparticle is increased. The results of cyclic voltmeter for 0.5gm-1 zinc oxide is illustrated in the fig.6. |
 |
IV. CONCLUSION |
| In the present study the Zinc Oxide Nanoparticles and Collagen based thin film Sheet generates current of 4 μA and 4 mA.The result shows that as the concentration of the zinc oxide nano particle increases there is a marginal change in the current output. The resulting of Biosensor strips for the experiments in response to the 2.8mM solution and 16.7mM solution. The voltage peaked at 280mv for the 2.8mM solution (hypoglycaemia) and 860mv for the 16.7mM solution (hyperglycaemia). The solution of higher concentration resulted in a higher spike in voltage. This is made as a bench scale model and further standardization and calibration is to be repeated for pilot scale production. The accuracy of the reading is less than 1 % error. The product is cost effective and further it can be reduced when it is made in a large scale. |
V. ACKNOWLEDGEMENT |
| Authors thank the Director, CLRI, for the continuous support and encouragement. |
References |
|