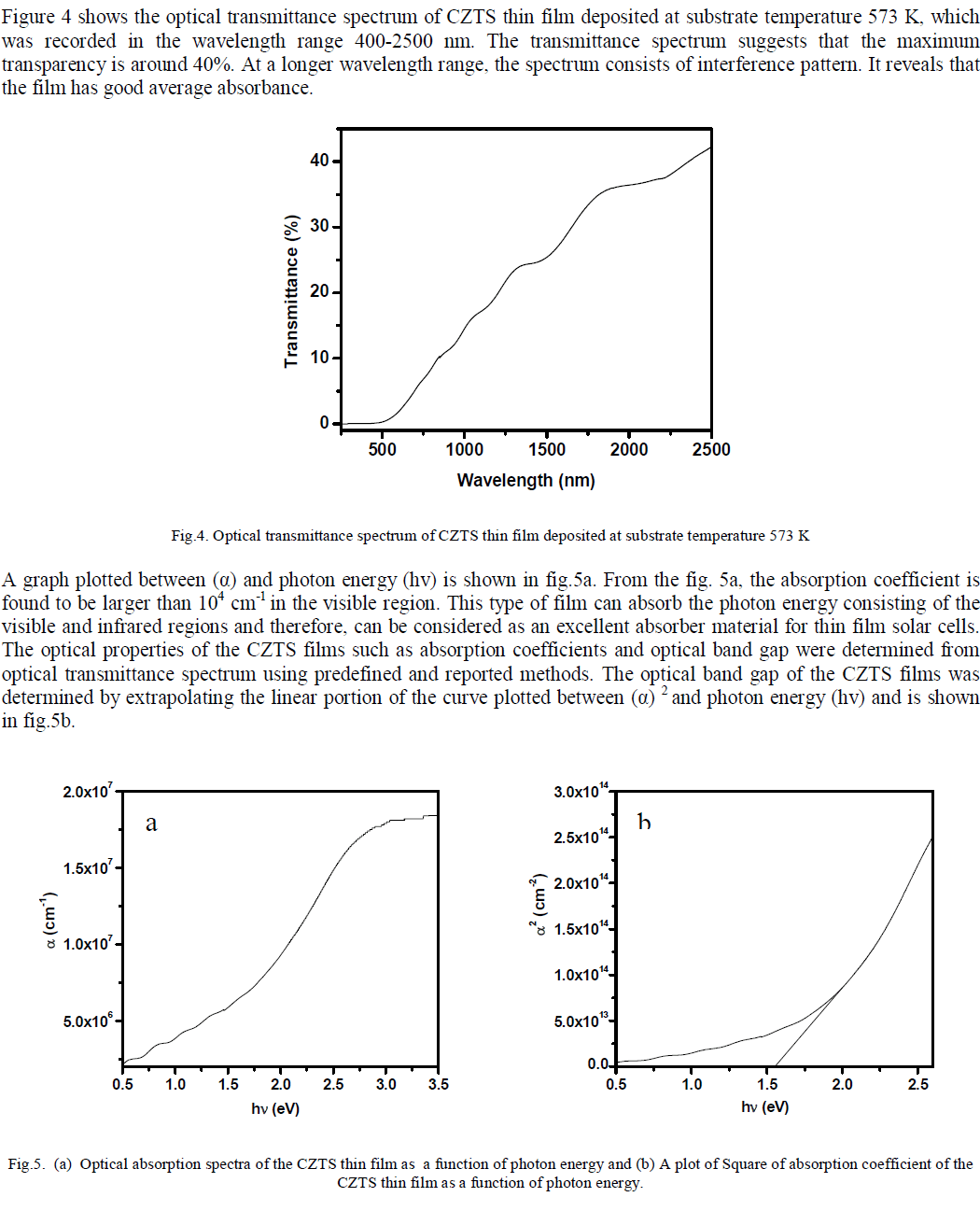
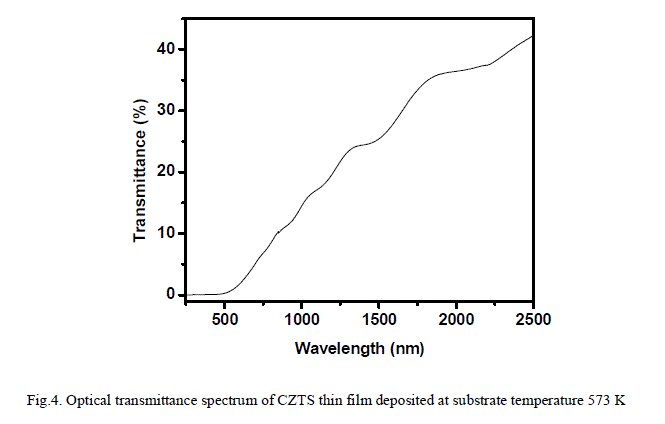
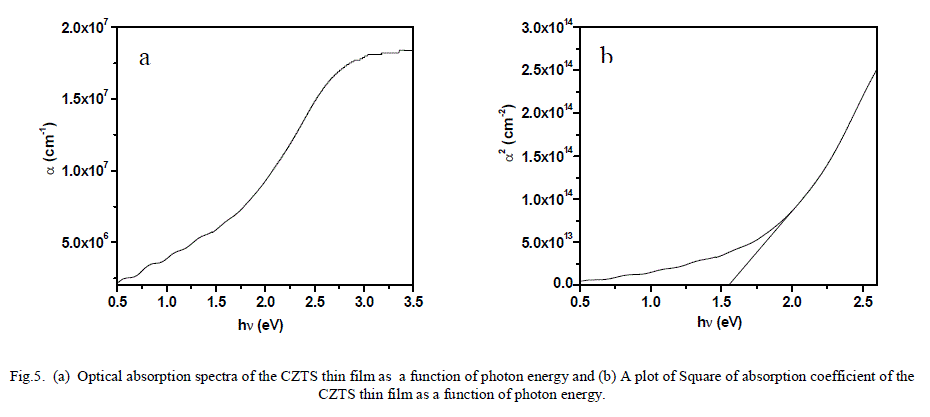
ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Ahmed A. Moosa, Ali Mousa Ridha, Ibtihal Najem Abdullha Department of Materials Engineering Technology, Engineering Technical College– Baghdad, Middle Technical University Baghdad, Iraq |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Functionalized MWCNTs were used for the removal of heavy metal Cr+3 ions from wastewater. The optimum conditions for batch adsorption of Cr3+ ions and the maximum adsorption efficiency were found to be 99.83% for MWCNTs at 25 mg; and pH 6. The results showed that MWCNTs have large adsorption capacity due to their higher surface area. The equilibrium isotherm of (MWCNTs) shows that the Langmuir isotherm fits the experimental data better than Freundlich adsorption
Keywords |
| Adsorption, Heavy metals, Heavy metal Removal, Activated Bentonite. |
INTRODUCTION |
| Heavy metals in natural or industrial wastewaters are a subject of great interest in environmental science which is one of the most serious worldwide environmental problems. These heavy toxic metals entered into the water bodies through waste water from metal plating industries and factories of batteries, mining, and pigments [1]. |
| Chromium is a heavy metals and present in the environment in several different forms. The most common forms chromium (III), and chromium (VI). The sources of Cr are wastewaters of electroplating, leather tanning, cement, and paint industries [2-3]. Chromium removal techniques such as precipitation, membrane techniques and solvent extraction are challenged by the removal of lower concentrations of metals from solution. On the other hand, adsorption has been proven to be one of the respective methods, which is simple, selective andeconomical process for the removal of heavy metal ions from aqueous solution [4]. |
| Adsorption on smectite-rich clays, i.e., bentonites, is a reliable method of metal removal owing to its simplicity, effectiveness, low cost, and bentonites are abundant [5]. The removal of metal ions using bentonite is based on ion exchange and adsorption mechanisms due to its relative high cation exchange capacity (CEC) and specific surface area. Bentonite holds pollutants and prevents downward movement of wastewater. Hence, it is important to know the retention capacity of bentonite and the factors that govern cation retention [6]. The adsorption of the chromium on bentonite applied for the successful removal of Cr (III) from tannery wastewater [3]. |
| The aim of this work is to use raw and modified bentonite as low cost adsorbents materials for the removal of Cr+3 in aqueous solutions. The effect of process parameters such as: the dosage of adsorbent, pH, and type of adsorbent on the adsorption process will be investigated. |
MATERIALS AND METHODS |
Adsorbent |
| The adsorbent used was Iraqi Calcium bentonite( Ca B) supplied by Iraqi National Company for Geological Survey and Mining, Baghdad. The chemical composition of calcium bentonite (CaB) is presented in Table1. |
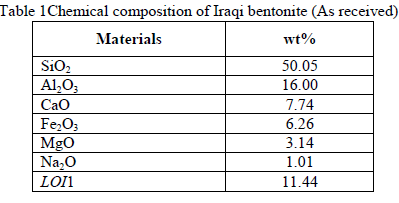 |
Adsorbate |
| A 1000 mg/L standard stock solution of Cr3+ions was prepared by dissolving 7.692 g of chromium nitrate salt (Cr (NO3)3.9H2O, 99% purity, Escrow, China) into (1000mL) of deionized water and stirred using magnetic stirrer. |
Natural Bentonite |
| As received bentonite was washed with deionized water several times and then filtrated under vacuum using filter paper in order to remove suspended insoluble impurities and then dried at 70oC for 3 hrs using drying furnace. The dry bentonite was then sieved to a < 75 micron particle size and then stored in desiccator for further use. Throughout this work this is called Raw Bentonite (RB). |
Thermal Activated Bentonite |
| Raw bentonite (RB) was then modified by thermal activation at different temperatures from (100, 200, 300, 400 500, and 700oC) for 3 hrs. |
Acid Activated Bentonite |
| A 25 g of raw bentonite was then acid activated with H2SO4 acid (GCC, British) of different concentrations (0.5, 0.75, 1, 1.5, 1.75 and 2M). The acid to raw bentonite ratio is fixed at 1:10 i.e. a 25g of raw bentonite was mixed with 250mL of known concentration of H2SO4 solution using magnetic stirring for 3hrs. After the reaction was terminated, the bentonite was washed many times with a deionized water to remove excess acid and pH 7 was obtained. After washing and filtration, the bentonite was dried at 70oC for 3hrs. This is called acid activated bentonite (AAB). The bentonite was then sieved to <75 μm particle size and then stored in desiccators for further use. |
Acids and Thermal Activation Treatment of Bentonite |
| The combined acid and thermal activated bentonite treatments are two-step procedures. In the first step, the optimum measured parameters of the acid activated bentonite (such as surface area, pore volume, Functional groups) was taken and then followed by thermal activation. Each 25g of raw bentonite was acid activated with 250mL of known optimum concentration of H2SO4 solution. The suspension was mixed using a magnetic stirring for 3hrs and then washed with large amount of deionized water to remove excess acid until the solution is neutralized pH 7. After washing and filtration, the bentonite sample was dried in a furnace 70oC for 3hrs and then thermally activated by heating at optimum known temperature for 3 hrs in a furnace. The bentonite was cooled in the furnace and then sieved again to < 75 μm particle size and stored in the desiccators for further use. |
Characterization of Modified Bentonite |
| The specific surface area and pore size of AAB and TAB and combined AAB/TAB bentonite was measured using the BET analyzers, model 9600, USA. The Fourier-Transformation Infrared Spectra (FT-IR) were recorded on Thermo- Nicolet 370, (USA) infrared spectrophotometer. |
Effect of Adsorbent weight |
| The removal of Cr3+ from aqueous solution onto raw and modified bentonite was achieved in a batch adsorption system. To determine the optimum weight of the RB and modified bentonites for removal of Cr3+ ions, different weights (5, 15, 25, 50, 75,100, 200, 300, 400 mg) of modified bentonite were mixed with 50 mL of chromium ions solutionCr3+. The effect of amount of adsorbent (bentonite) was investigated by using initial concentration of 100 mg/L of Cr3+ ions solution at pH 7. The desired pH was adjusted using 1 M HCl and 1 M NaOH. The bentonite solution bottles were mixed for about 3hrs in a rotary shaker at 140 rpm and 25oC. The solution was then filtered using and filter paper under vacuum and the remaining of Cr3+ ion concentrations in the filtrate were measured by using the atomic-absorption spectrophotometer. The optimum weight of RB, AAB, TAB and the combination AAB/TAB bentonite at pH 7 was then selected. |
Effect of pH |
| To obtain the optimum pH of Cr3+ ion solution adsorption onto RB, AAB, TAB and AAB/TAB , a 50 mL of Cr3+ metal ions solution at initial concentration of 100 mg/L was used at different pH ( 2, 4, 6, 8, 10). The pH of each samples was adjusted by 1M of concentrated HCl acid and 1M NaOH by pH meter. The metal ion solutions Cr3+ of different pH was added to the bottles containing optimum weight of RB, AAB, TAB, and combination AAB/TAB bentonite. The bottles were then shaken in a rotary shaker for 3 hrs at 140 rpm and 25oC. The bentonite solution was then filtered using filter paper with vacuum. The remaining of Cr3+ ion concentrations in the filtrate were determined by using the atomic-absorption spectrophotometer. The optimum pH of RB , AAB ,TAB and the combination AAB/TAB bentonite were then selected. |
Equilibrium Isotherms |
| Equilibrium isotherms experiments were carried out using the optimum weight of all the adsorbents and the optimum pH of Cr3+ metal ion solutions for each adsorbents. A 50 mL of Cr3+ ion solution of different initial concentration (20, 40, 60, 80, 100, and 120 mg/L) was added in bottles containing the optimum adsorbent weight and the optimum pH of solution. The bottles were then shaken at a fixed speed of 140 rpm in rotary shaker water bath at different temperatures of 25, 35 and 45oC for 3hrs. The solution was then filtered as before and the remaining Cr3+ ion concentrations in the filtrate were determined by using the atomic-absorption spectrophotometer. |
| The adsorption capacity, qe (i.e the mass of solute adsorbed per mass of adsorbent) was then plotted versus the equilibrium concentration of the solution, Ce to obtain the equilibrium isotherm curves. |
| The adsorption of metal ions onto the adsorbent surface at specific time was estimated from mass balance equation [7]: |
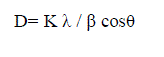 |
 |
RESULTS AND DISCUSSION |
FTIR |
| The Fourier transform infrared spectroscopy (FTIR ) of the raw and activated bentonites were carried out in the range 400- 4000 cm-1. The absorption bands of raw bentonite at 3614.60 cm-1 is due to vibrations of the OH groups for the water molecules adsorbed on the clay surface. The band at 3436 cm-1 is due to OH-vibration of physically adsorbed water which is close to 3430 cm-1 as reported by [14]. The bending vibration mode of water is 1635.64 cm-1. A band at 1037.70 cm-1 is assigned to the stretching vibration of Si-O groups. The bands corresponding with the Al-Al-OH bending vibrations were observed at 918.12 cm-1 which is close to 913 cm-1 reported by [15]. The Al-Mg-OH bending vibrations were observed at 833.25 cm-1 which is close to 835 cm−1 reported by [16]. The bands at 528.50 and 478.35 cm-1 correspond to the bending vibrations of Al-O-Si and Si-O-Si, respectively. |
| The FTIR spectra of acid activated bentonite is shown in Figure 1a. During acid-leaching of the clay , the OH groups will be attacked by the protons from the acid media causing the alteration in the adsorptive bands attributed to the OH vibrations and octahedral cations. The peak associated with the bending vibration mode of water at 1625.64 cm-1 and decreases after acid activation due to the removal of octahedral cations, causing the loss of water. The bands at 528.50 and 478.35cm-1 correspond to bending vibrations of Al-O-Si and Si-O-Si, respectively disappeared after the acid activated bentonite. This is in agreement with the work of [17]. |
| The FTIR spectra of thermally activated bentonite at 200 oC is shown in Figure 1b. The band at 3436 cm-1 did not disappear on heating and was correlated with structural OH groups. The intensity of the bending vibration mode of water is 1454.21cm-1. A band at 1033.34 cm-1 is decreased which is assigned to the intensity of the stretching vibration of Si-O groups. Further heating, the shape of many peaks was changed. However the structure of bentonite leads to the formation of pores as a result of eliminating of OH-groups from the interlayer space and the bentonite collapsed at high temperature.This is in agreement with the result obtained by [18]. |
| For acid and thermal activated bentonite (AAB/TAB) FTIR spectrum , the increase in the activation temperature resulted in the deeper penetration of protons into the clay layers. The intensity of many peaks decreases. There are a decrease in the intensity of the peaks in the bands at 3630.03 cm-1 associated with adsorbed water ( H-O-H stretching) and the band at 1635.64 cm-1 (H-O-H bending). The bands at (524.64, 474.49) cm-1 due to bending vibrations of Al-OSi and Si-O-Si, decreased with the increase in temperature due to the dissolution of Al ions that present in the octahedral sheet of bentonite. This is in agreement with the result obtained by [19]. |
 |
Surface Area |
| Figure 2 shows the surface area of acid activated bentonites (AAB) using different concentrations (0. 5, 0. 75, 1, 1.5, 2) M of H2SO4 solution at room temperatures using BET analysis. The surface area of raw bentonite (RB) is 22.43 m²/g. The surface area of AAB bentonite increases with increasing H2SO4 acid concentration until a maximum surface area is reached after which it gradually decreases. The maximum surface area (32.68 m2/g) occurs at 1.5M H2SO4 acid concentration. This increase is attributed to dissolution of impurities; the leaching of Al3+, Fe3+ and Mg 3+ from the tetrahedral and octahedral sites which exposes the edges of AAB particles and the replacement of cations (K+, Na+, Ca2+ ) with hydrogen ions [17]. |
| The decrease in surface area at higher H2SO4 acid concentrations is caused by deep penetration of the acid into the voids and the excessive leaching of Al3+, Fe3+ and Mg 3+. This will lead to the collapse of layered structure and close packing of particles [20]. |
| The surface area of the thermally activated bentonite (TAB) at different temperature (100,200,300, 400,500, and 700)oC is shown in Figure 3. The maximum surface area of TAB is (27.61 m²/g at 200oC). At temperature greater than 500oC the surface area remains constant. At high temperature (greater than 200oC but less than 500oC) decomposition of structure and breakdown of the bonds within the clay structure resulting in the collapse of structure and decrease in surface area in agreement with the work of [21]. |
| The acid and thermal activated bentonite treatments (AAB/TAB) are two-step procedures. The acid activated bentonite with the maximum surface area at 1.5M H2SO4 concentration was taken and then followed by thermal activation process at the optimum temperature of 200oC. The BET analysis of AAB/TAB shows the maximum surface area 109.85 m²/g. |
 |
Weight of Bentonite |
| The effect of RB and activated bentonites weight on the adsorption process are shown in Figure 4. The adsorption efficiency of different weights (5, 15, 25, 50, 75,100, 200, 300, 400 mg) of raw bentonite mixed with 50 mL of Cr3+ solution at 7 pH is shown in Figure 4. The result indicates that the adsorption efficiency increases with weight until equilibrium is reached. Initially, the increases in adsorption efficiency may be explained by an increased availability in the number of active binding sites on the adsorbent surface. The maximum removal occurs at bentonite weight of 100 mg. After that the adsorption is constant due to the fact that every adsorbent has a limited number of active sites which becomes with time occupied with Cr3+. This is in agreement with the work of Badmus et el. [22]. The adsorption efficiency is 99.839% for raw bentonite. Initially , increasing the adsorbent weight means a greater number of available adsorption sites therefore, the ratio of number of adsorption sites to the number of heavy metal ions will increase [23]. |
| The acid activated bentonite experiments were carried out at 1.5 M H2SO4 (the optimum surface area, 32.68m2/g). Figure 4 shows the results of the adsorption capacity at 7 pH using different weights (5, 15, 25, 50, 75,100, 200, 300, 400 mg) of AAB mixed with 50 mL of chromium ions solutionCr3+. The adsorption efficiency increases with increasing the weight of adsorbent until equilibrium was reached. The maximum adsorption efficiency of AAB is 99.802 % at 50 mg . The AAB weight was smaller than that of raw bentonite , while the specific surface area was more than that of raw bentonite. This is due to modified structure and functional groups . |
| The thermal activated bentonite experiments were performed at 200oC (the optimum surface area, 27.61m²/g). Figure 4 shows the results of the adsorption efficiency at 7 pH using different weights (5, 15, 25, 50, 75,100, 200, 300, 400 mg) of TAB mixed with 50 mL of Cr3+ solution. The maximum adsorption capacity of TAB was 99.84 % at 75 mg. This could be explained according to the availability of adsorption sites for removal of Cr3+ ions onto TAB, comparing with the availability of adsorption sites in raw bentonite. |
| The combined acid and thermal activated bentonite experiments were performed at 1.5M H2SO4 of AAB (the optimum surface area, 32.68 m2/g) followed by thermal activation at 200oC (the optimum surface area reached , 27.61 m²/g) using different weights (5, 15, 25, 50, 75,100, 200, 300, 400 mg). The AAB/TAB bentonite gives maximum surface area of 109.85 m²/g. Figure 4 shows the maximum removal adsorption is 99.838% at 25mg of AAB/TAB. Further increase in weight indicates that the adsorption mechanism is reversible and that there exists chemical equilibrium between adsorbent and adsorbate [24]. |
| Higher adsorption sites are available at lower AAB/TAB weight. The order of the increase in surface area is AAB/TAB > AAB >TAB >RB. |
 |
Effect of Chromium Solution pH on The Adsorption |
| The effect of pH on adsorption of Cr3+ ions was investigated by varying the pH from 2 to 10. Figure 6 shows the adsorption capacity for RB increases for pH (2-6) due to decrease in competition between H+ and Cr3+ and decrease in positive surface charge, which results in a lower electrical charge between the particle surface of bentonite and Cr3+ ions. The maximum adsorption efficiency (97.542%) for RB occurred at the pH 6. The decrease in the Cr3+ ions removal capacity at pH > 6 may be caused by the formation of complexes between Cr3+ and OH- . This Cr hydroxyl may participate in the adsorption and precipitate onto the RB bentonite structure [25]. |
| The maximum adsorption efficiency (99.01%) occurred at pH 6 for AAB as shown in Figure 5. The adsorption efficiency increases between pH 2- 6 due to the presence of more negatively charged surface on acid activated bentonite. The decrease in the Cr3+ ions adsorption efficiency at pH > 6 caused by the formation of complex ion of Cr3+ with hydroxide in agreement with the work of A. Mellah, et al., [25].The increase in specific surface area of TAB will give higher adsorption efficiency due to the increase in active sites compared with (RB) bentonite. This will lead to increase in the removal of Cr3+ ions. The maximum adsorption capacity (98.643 %) occurred at pH 6 as in Figure 5. The decrease in the Cr3+ ions adsorption efficiency at pH > 6 caused by the by the formation of complex ion of Cr3+ with hydroxide in agreement with the work of [25]. |
| The adsorption efficiency of Cr3+ ions increases with increasing the higher active sites of surface area of (AAB/TAB) bentonite. Figure 5 shows for (AAB/TAB) the higher adsorption efficiency (99.88 %) occurred at the pH 6. It was found that the adsorption efficiencies are in the following order: AAB/TAB > AAB >TAB > RB bentonite . |
Equilibrium Isotherm of Raw and Modified Bentonite. |
| The experimental results of the adsorption isotherm curves were obtained by plotting the weight of the metal adsorbed per unit weight of the adsorbent against the equilibrium concentration of the solute (metal). The adsorption efficiency of the metal adsorbed were determined using Eq.(2.1). Figures 6 - 7 show the adsorption isotherm curves for Cr+3 ions onto RB, and AAB/TAB at 25 oC respectively. The experimental data for Cr+3 adsorption onto activated bentonite are correlated with the two theoretical adsorption isotherm models. These Figures describe the experimental data and the theoretical data obtained from Langmiur and Freundlich isotherm. All constants and correlation coefficients for Langmiur and Freundlich isotherm theoretical model are listed in Table 2. |
| Figure 6 shows that the adsorption capacity of Cr+3 metal ions onto RB increases with increasing the solution concentration. This is in agreement with work of [26]. The initial adsorption rates were high because at the start of adsorption all the adsorption sites on the adsorbent were vacant. After that the adsorption sites were filled up and the metal solution concentration decreased until equilibrium was reached [27]. |
| The value of the correlation coefficient R2 from Table 2 indicates that Langmuir isotherm fit the experimental data better than Freundlich adsorption isotherm for raw bentonite. |
 |
| The data at optimum condition ( 1.5 M H2SO4 , surface area, 32.68m2/g, 50 mg) of acid activated bentonite were used at different Co (20, 40, 60, 80, 100,120 mg/L) to construct the experimental model and the Langmuir and Freundlich isotherm at 25 oC. It can be concluded that the Langmuir model yields better fit to the experimental data than the Freundlich model. |
| The data at optimum condition surface area (27.61 m2/g, 200oC ,75 mg) of thermal activated bentonite were used at different Co (20, 40, 60, 80, 100,120 mg/L) to construct the experimental model and the Langmuir and Freundlich isotherm at 25oC .These data were then used in Table 2. Based on correlation coefficient R2 (from Table 2) it can be concluded that the Langmuir model yields better fit to the experimental data than the Freundlich adsorption isotherm. |
| The data at optimum condition (surface area 109.85 m²/g , 25mg) of the AAB/TAB activated bentonite was used at different Co (20, 40, 60, 80, 100,120 mg/L) to construct the experimental model and the Langmuir and Freundlich isotherm at 25oC . These data were then used in Figure 7 and Table 2. Based on correlation coefficient R2 (from Table 2) it can be concluded that the Langmuir model yields better fit to the experimental data than the Freundlich adsorption isotherm. |
| The values Langmuir constants b , qm and correlation coefficient R2 increased at 45oC due to high improved of structure by its extended surface area and high degree of surface reactivity, arrange in the following order: AAB/TAB > AAB >TAB > RB bentonite. |
| The effect of different temperatures (25, 35, 45 oC) on the adsorption isotherm of Cr+3 ions onto raw and modified bentonite was investigated. The results from Table 2 show that the Langmiur adsorption isotherm gives the best fit to the adsorption isotherm for the temperatures (25, 35, 45 oC). The adsorption capacity increases as the temperature increases, and this may be attributed to activation energy of adsorption. Also, this behavior could be related to increase in the available number of active surface sites as reported by [28]. |
 |
Thermodynamic Parameters of Modified Bentonite |
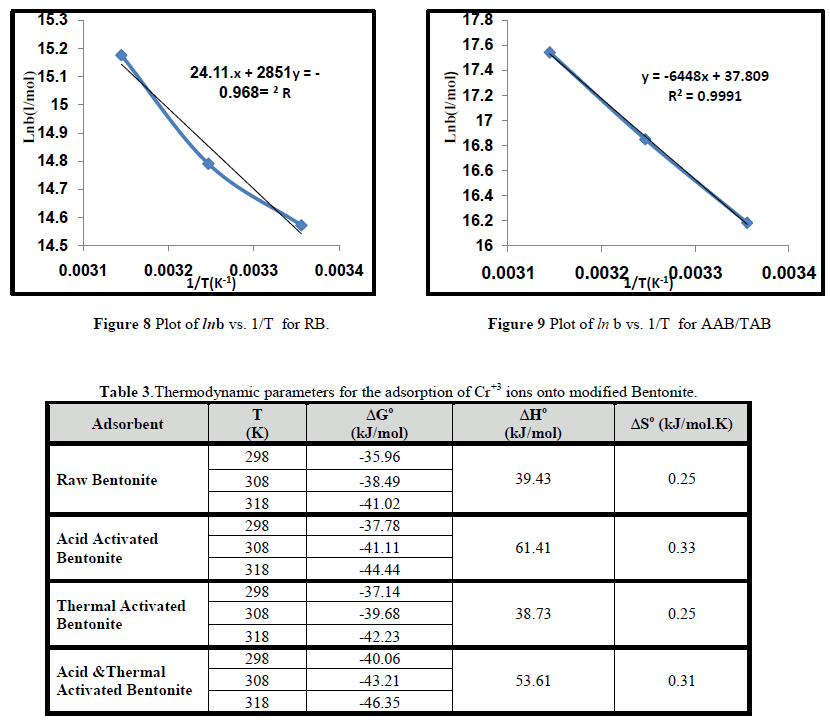
| The thermodynamic parameters for adsorption process such as free energy ΔGo, enthalpy ΔHo, and entropy ΔSo were calculated using Eq 7 &8 [10] . |
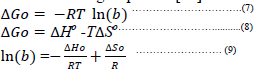 |
| The values of enthalpy ΔHo and entropy ΔSo were obtained from the slope and the intercept of a linear plot between lnb against 1/T from Eq. (9) as shown in Figures 8-9 for adsorption Cr3+ ions onto RB and AAB/TAB respectively . The results are tabulated in Table 3 for RB and activated bentonites. The values of ΔGo decrease slightly with increases in temperature. This behavior indicates that the reaction is spontaneous and more favorable at higher temperature. At higher temperature the ions are desolvated and thus their adsorption becomes more favorable as reported by [29]. The positive values of ΔHo from Table 3 indicates the behavior of the adsorption of Cr+3 ions is endothermic in nature and a small amount of energy is required to transfer the Cr+3 ions from aqueous into the solid phase process. The positive values of ΔSo indicates an increase in the degree of randomness at the solid-solution interface which is in agreement with the work of [30]. |
 |
CONCLUSION |
| Iraqi betonies is a cheap and effective adsorbent for removing of Cr3+ ions from wastewater. Modified bentonite by acid (AAB) , thermal (TAB) and thermal/acid (TAB/AAB) treatment gave better Cr3+ removal than raw bentonite. For modified bentonites , the (AAB/TAB ) has the maximum surface area 109.85 m²/g . The maximum adsorption efficiency for modified bentonites (AAB/TAB) is 99.83% at 25mg and pH 6 but decreases at pH > 6 because of the complex Cr3+ ions with hydroxide. The equilibrium data described by the Langmuir and Freundlich isotherm equations showed that the Langmuir isotherm fit the experimental data better than Freundlich adsorption isotherm for raw bentonite and modified bentonite at temperature (25,35,45 oC). The thermodynamic results of modified bentonite showed negative Gibbs energy. The positive values of ΔHo indicates endothermic and the positive values of ΔSo indicating increased in the randomness with increased temperature during adsorption process. |
References |
|