e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
1Department of Pharmaceutical Sciences, Health Sciences Center, Federal University of Santa Catarina, 88040-900, CP 476 Florianópolis, SC, Brazil
2Pharmacy Course, University of Contestado, Campus Canoinhas, 89460-000 Canoinhas, SC, Brazil
3 Pharmacy Course, University of the West of Santa Catarina, Campus Vine, 89560-000-SC, Brazil
4Center for Chemical-Pharmaceutical Research (NIQFAR), University of Vale do Itajaí (UNIVALI), 88302-202 Itajaí -SC, Brazil
5Department of Veterinary Medicine, University of the State of Santa Catarina, University Campus of Lages, 88520-000 Lages, SC, Brazil
Received date: 21/05/2015 Accepted date: 10/07/2015 Published date: 13/07/2015
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Antitumor and antimetastatic effects of N-Phenylmaleimide derivatives (N-phenyl-maleimide (M2), 4-methyl-N-phenyl-maleimide (M5), 4-methoxy-N-phenyl-maleimide (M7 and N-phenyl-ethyl-maleimide (M9)) were assessed on mice implanted subcutaneously with B16F10 melanoma cells. With this approach, we evaluated the growth and persistence of solid tumors, lung metastasis, mesentery metastasis, biochemical and hematological parameters, thiobarbituric acid reactive substances (TBARS) and non-protein thiol substances (NPSH) present in the liver homogenates. The N-Pheneylmaleimides reduced the number of lung metastasis and inhibited tumor growth. The treatments with M5 and M7 showed low toxicity because minor alterations in hematological and biochemical parameters were observed, and only the treatment with M9 promoted intense oxidative stress as well as alterations in some hepatic enzymes. The effects of maleimides shown in this work can be related to their chemical structure features, principally by the proximity between the imide and aromatic rings, and the hydrophobic and electronic characteristics of the substituent groups on the aromatic ring, which can modify their lipophilic properties. These preclinical results indicate that treatments with M5 and M7 may be effective against melanoma suggesting that these compounds may be further used as leads to develop new anticancer agents.
Melanoma, maleimides, subcutaneous tumor, tumor growth.
Melanoma is a malignant tumor of melanocytes that predominantly occurs in the skin of Caucasians worldwide. Primary melanoma, once detected, should be surgically removed, and chemotherapy and radical regional lymphadenectomy should focus on metastasis control [1]. The leading cause of death in advanced melanoma patients is the formation of metastasis at distant sites. The most frequent metastasis sites for melanoma are the small bowel, liver, bone, brain and lung [2]. An evidence-based overview has shown that dacarbazine in monotherapy is the least harmful treatment against melanoma [3].
The alkaloid phyllanthimide isolated from leaves of Phyllanthus sellowianus (Euphorbiaceae) has been used as a precursor for the synthesis of many analogs belonging to the class of cyclic imides [4]. Cyclic imides are organic compounds formed by the group CO-N(R)-CO-, R being a hydrogen atom from the alkyl or aryl group, which confers potential biological activity and pharmaceutical applications [5]. Some of these effects appear to be related to the size and electrophilic characteristics of the substituent groups on the imide ring, which can modify their steric properties. The N-phenylmaleimides contain an imide ring that confers hydrophobicity and neutral characteristics to the molecules [6].
The literature contains several studies on the antitumor potential of maleimides. The antiproliferative activity of a series of heteroarylmaleimides and polyheterocondensed imides was tested against human tumor cells (NCI-H460 lung carcinoma), and the IC50 values obtained ranged from 0.84 to 9 μM [7]. A series of 7-azaindazolyl-indolyl-maleimides and arylmaleimide derivatives show cytotoxicity and antiproliferative effects in vitro against various human cancer cell lines: K562 and HL 60 (leukemia), A549 (human lung carcinoma), ECA-109 (human oesophageal carcinoma), KB (human epidermoid carcinoma), SMMC-7721 (human hepatocellular carcinoma), PC-3 (human prostate), and SGC-7901 (human gastric). These works also show moderate protein kinase C inhibitory activity and the possible involvement of the mitochondrial-mediated pathway of apoptosis in the antiproliferative activity of some maleimides on KB cell [8,9].
However, N-Phenylmaleimides, to our best knowledge, have never been studied against melanoma in vivo. Firstly four maleimides including, N-phenyl-maleimide (M2), 4-methyl-N-phenyl-maleimide (M5), 4-methoxy-N-phenyl-maleimide (M7), and N-phenyl-ethyl-maleimide (M9) (Table 1) were evaluated in vitro. As the compounds showed toxicity in vitro, they were tested in a preclinical a mouse melanoma model.
Materials
The cell culture media, serum and antibiotics were purchased from GIBCO (São Paulo, Brazil). Dimethyl sulfoxide (DMSO) and ethylenediaminetetraacetic acid disodium salt (EDTA) were purchased from Merk (Darmstad, Germany). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide) was purchased from Invitrogen. HEPES [N(-2-hydroxyethyl)piperazine-N’-(2- ethanesulfonic acid)] sodium salt, Trypan blue, trypsin, DTNB [5,5’-dithiobis(2-nitrobenzoic acid)], and TBA (thiobarbituric acid) were purchased from Sigma Chemical Co. (St. Louis, MO, USA), and all reagents were commercial products of the highest available purity.
Compounds
The four N-phenylmaleimide studied were synthesized at NIQFAR - University of Itajaí Valle (UNIVALI), Itajaí, SC, Brazil. Their structures were confirmed by conventional spectroscopic methods, according previously described [5,10]. The synthesis of the N-phenylmaleimide derivatives was achieved by the reaction of maleic anhydride with the appropriate amine in the presence of glacial acetic acid at room temperature, which generated the corresponding N-substituted maleamic acids. The maleamic acid intermediates were subsequently cyclized to the corresponding N-arylmaleimides by heating in the presence of acetic anhydride containing catalytic amounts of sodium acetate [10,11]. The structure of maleimides (M2, M5, M7 and M9) was shown in Table 1.
The compounds were solubilized in DMSO and used in different concentrations. Controls were run in parallel to monitor the possible interference of DMSO with the assays. The final DMSO concentration was 0.1% in the in vitro assays and 2% in the in vivo assays. In these conditions, the solvent did not interfere with the assays.
Tumor cell line
Tumor cell line of B16F10 murine melanoma was acquired from ATCC, Manassas, VA. The cells were cultivated in cell culture flasks of 75 cm2 in DMEM supplemented with 10% of inactive fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 10mM HEPES (pH 7.4). Every 2-3 days, cells were passaged by removing 90% of the supernatant and replacing it with the fresh medium. The cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C and then harvested with trypsin: EDTA (0.05:0.03 w/v) solution.
Evaluation of cytotoxicity
The cell viability with or without the compounds was measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], a colorimetric assay [12]. This assessment of cytotoxic activity is based on the ability of living cells to reduce the yellow product MTT to a blue formazan product. The B16F10 cells were incubated for 24 and 48 h in 96-microwell plates (at a density of 3 x 104 and 1.5 x 104 cells/0.2 ml, respectively) with maleimides in a concentration range of 10 to 100 μM. Next, the cells were carefully washed, and a MTT solution prepared in fresh medium was added and further incubated for 2 h at 37 °C and 5% CO2. The medium was removed, and DMSO was added to dissolve the crystalline formazan formed. The number of living cells is directly proportional to the intensity of the blue solution formed, which is quantitatively measured by a microplate reader at 550 nm wavelength. The results were expressed as a percentage of the absorbance of the control. Cell viability of the control cells corresponds to 100%, and half of the inhibitory concentration is called IC50. All experiments were performed in triplicate.
Mitochondrial potential measurements
To evaluate the effect of maleimides on the mitochondrial membrane potential, the lipophilic cationic probe fluorochrome 5,58,6,68-tetraethylbenzimidazolcarbocyanine iodide (JC-1) was used. JC-1 is a green fluorescent monomer at depolarized membrane potentials or a red fluorescent J-aggregate at hyperpolarized membrane potentials. Cells were plated at 5 x 105 cells/well in 24-well dishes and treated with the maleimides for 1 h. Afterward, JC-1 (10 μg/ml) was added and incubated for 20 min at 37°C(5% CO2). The cells were washed twice with PBS, resuspended in PBS, and an aliquot was used to measure the fluorescence in a spectrofluorometer (Perkin Elmer LS55). The excitation wavelength for JC-1 is 488 nm, and the red and green emission fluorescence was detected at 590 nm and 527 nm, respectively [13]. The mitochondrial potential was presented as a ratio of 590/527 fluorescence and compared with the control. An electron transport chain uncoupler (FCCP 1 μM) was used as a positive control.
Subcutaneous in vivo model
The maleimides were dissolved in 100% DMSO and diluted in PBS at a final concentration for injection in each animal (maleimides=0.27 mg/kg body weight). Swiss female mice, weighing around 25 g each and ageing from 6 to 8 weeks were used in the in vivo experiments. The mice were maintained under specific pathogen-free conditions. The animals were separately housed in ventilated cages under a controlled light cycle (12 h light/12 h dark) at a standard room temperature(22-24°C)and a relative humidity of 50-60% and were allowed access to a conventional diet and water ad libitum during entire experiment period. The animals were kept under these conditions for at least one week before the experimental procedures.
The experiments with animals were performed in accordance with ethical guidelines of the Helsinki Declaration (1975) and were previously approved by the University’s Ethics Committee for Animal Use on the Principles of Animal Care.
The cell suspension was detached from flasks with trypsin. Upon trypsin inactivation with DMEM containing 10% fetal bovine serum, the cells were centrifuged and resuspended with PBS. The viable cells were counted and the cell suspension was diluted at a final density of 1.25 x106cells/ml.
Tumor formation was performed in mice by subcutaneous inoculation in dorsal region of 2.5x105 B16F10 cells suspended in 0.2 ml of phosphate-buffered saline [2]. This procedure was done in all animals except in the animals of the group 1 (negative control group) and groups 3, 5, 7, and 9, which correspond to four groups (without tumor) to explore the inherent toxicity of maleimides.
Fourteen days after tumor cell injection, when a solid mass appeared (3 mm diameter), the animals were divided in five groups with ten animals each (groups 2, 4, 6, 8, and 10 depending on the treatment).
All animals were weighed before and after treatments. The groups’ distribution is detailed as follow: the treatment was administered in alternate days for one week (four administrations), by intraperitoneal route. The doses were calculated considering the minor concentration evaluated in vitro assay with some maleimides reported by Prado et al. [6] that corresponds of about twenty times more than the IC50 for cytotoxicity for M2.
The groups were named as follow: Group 1 (G1): The negative control received only the vehicle (2% DMSO in PBS); Group 2 (G2): Ten dorsal melanoma-bearing animals treated with vehicle (positive control); Group 3 (G3): Five animals treated with the solution of M2 (0.27 mg/kg body weight); Group 4 (G4): Ten dorsal melanoma-bearing animals treated with the solution of M2 (0.27 mg/kg body weight); Group 5 (G5): Five animals treated with the solution of M5 (0.27 mg/kg body weight); Group 6 (G6): Ten dorsal melanoma-bearing animals treated with the solution of M5 (0.27 mg/kg body weight); Group 7 (G7): Five animals treated with the solution of M7 (0.27 mg/kg body weight); Group 8 (G8): Ten dorsal melanoma-bearing animals treated with the solution of M7 (0.27 mg/kg body weight); Group 9 (G9): Five animals treated with the solution of M9 (0.27 mg/kg body weight); Group 10 (G10): Ten dorsal melanoma-bearing animals treated with the solution of M9 (0.27 mg/kg body weight).
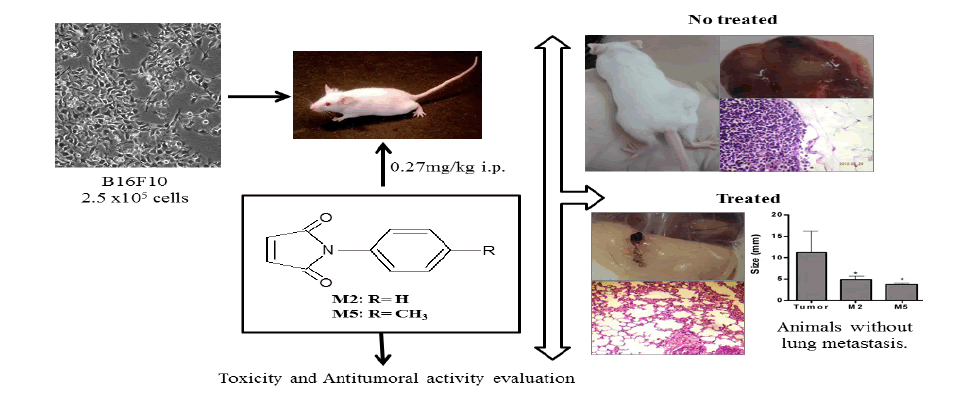
The day after the last treatment the animals were anesthetized and blood was collected from the ocular puncture vein into heparinized propylene tubes for hematological and biochemical analysis. Subsequently, the animals were killed and all organs excised and fixed with a 10% formaldehyde solution for histopathological analysis. A portion of liver was reserved under refrigeration for biochemical analysis (TBARS and NPSH) on the same day.
The tumors were carefully removed and their sizes were measured with a pachymeter. The entire process was monitored by photo documentation, and the characteristics of the tumors were annotated, including angiogenesis process characterized by presence of numerous blood vessels around the tumor. The tumors were sent for histological analysis. The tumor growth inhibition was calculated using the following formula: percentage of inhibition (%) = 100 (A – B)/A, in which A= tumors average size of the control group (G2) and B= tumors average size of the treated group. The macroscopic tumor nodules present in the mesentery and lung were also counted and measured with a pachymeter.
Toxicity observation – biochemical and hematological analyzes
Toxicity indexes such as weight loss, behavior changes, and feeding patterns were continuously observed during the whole treatment. To clarify potential side effects in the treated mice, the organs tissues were assayed according to assay protocol described below.
Samples of blood were centrifuged at 3,500 rpm for 15 minutes. The remaining serum was frozen at -20°C for subsequent analysis. The biochemical parameters determined were: serum total protein (TP), aspartate aminotransferase (AST), alanine aminotransaminase (ALT), gamma-glutamyl transferase (GGT), urea and creatinine. For biochemical parameters evaluation an automated equipment BS200 and GoldAnalisa® system kits for diagnosis were used following the manufacturer’s descriptions.
Hematological analysis including total white blood cell count (WBC) and differential leucocytes counts and red blood cell count (RBC), hemoglobin concentration (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC) were analyzed using standard hematological method as described by Dacie and Lewis [14]. Hematocrit (Hct) was determined by micro-capillary centrifugation at 800 × g for 10 min and was evaluated with a calibrated scale after sedimentation of the red blood cells.
The levels of non-protein thiol substances (NPSH)
NPSH substances were determined as described by Ellman [15]. The tissues fractions were obtained after homogenization in 1 volume of 12% trichloroacetic acid followed by centrifugation. An aliquot of the supernatant was added to phosphate buffer (at final concentration of 800 mmol/l at pH 7.4) and 500 μmol/DTNB (Ellman’s reagent (5,5'-Dithiobis-(2-Nitrobenzoic Acid)). The color development resulting from the reaction between DTNB and thiols reached the maximum in 5 min and remained stable for more than 30 min. To calculate the amount of NPSH substances in the tissue samples, a standard curve using glutathione was made in parallel.
Determination of thiobarbituric acid reactive substances (TBARS) level
TBARS were determined in the supernatant by the method of Ohkawa et al [16], in which malondialdehyde; the major endproduct of fatty acid peroxidation reacts with thiobarbituric acid to form a colored complex. Briefly, samples were incubated at 100°C for 60 min in a medium containing 0.45% sodium duodecyl sulfate, 100 mM hydrochloric acid, 1.4 M acetic acid, at pH3.4 and 0.6% thiobarbituric acid. After centrifugation the reaction product was determined at 532 nm.
Histological studies
The organs and tumors, after being removed from the animals, were washed in PBS, fixed in 10% neutral buffered formaldehyde solution dehydrated in graded alcohol for 24 h and then processed according to histopathological protocols. The histological analyses were performed in 4 μm sections stained with hematoxylin and eosin and observed by a pathologist in a blind manner. Analyzing 80 randomly selected fields histological damages were identified with an Olympus BH2-RFCA microscope at a magnification of 200 or 400 X.
The data were expressed as the means ± S.E.M. All data were statistically evaluated by variance analysis. Post hoc comparisons of individual means with the controls were completed using the Dunnett multiple comparison test. Values of P<0.05 were considered significant.
Antitumoral activity of maleimides
In vitro evaluation of the cytotoxic activity
The four maleimides tested induced significant cytotoxicity in B16F10 melanoma cells, presenting relatively low IC50 values (low micromolar range) after progressive times of incubation; 24, 48 and 72 hours (Table 1). This analysis of cytotoxic activity is based on the ability of living cells to reduce the MTT majorly by mitochondrial dehydrogenases. As the compounds inhibited the MTT reduction by the cells, it indicates they are acting via mitochondria. The next step was then to investigate whether the compounds could interfere with mitochondrial membrane potential.
In vitro evaluation of the mitochondrial membrane potential
The mitochondrial membrane potential is an important parameter of mitochondrial function and is used as a marker of different cell death mechanisms, including apoptosis. A lipophilic cationic probe JC-1, was used to detect alterations in the mitochondrial potential in B16F10 cells treated with maleimides.
Maleimides significantly decreased the mitochondrial membrane potential at 10 μM concentrations (Figure 1),
Figure 1: Effect of maleimides on mitochondrial membrane potential in B16F10 cells. The mitochondrial membrane potential was analyzed using a JC-1 fluorescent probe. The cells were incubated with maleimides (10 µM) for 1 h and FCCP (1 µM). A decrease in the red/green ratio indicates a decrease in the mitochondrial membrane potential in comparison with the control. * P<0.05;** P<0.01.
Similarly to a carbonylcyanide-ρ-trifluoromethoxyphenylhydrazone (FCCP), a proton ionophore and potent uncoupler of oxidative phosphorylation in mitochondria. The loss of the mitochondrial membrane potential is the primary event related to cytotoxicity observed in the MTT assay.
In vivo evaluation of antimelanoma activity
All animals tolerated the subcutaneous injection of melanoma cells and intraperitoneal injections of maleimides. The mice grew and developed normally, and none died during the experiment. The time course of the assay was 21 days. On the first day, the mice were inoculated with melanoma cells, and after 15 days, there was a palpable tumor nodule in the dorsal region of the animals. The mice were separated in their respective groups, and the treatments were then initiated.
Macroscopically, the melanoma tumor showed a rounded or ovoid morphology with an irregular multinodular surface and well-defined by a thin capsule of conjunctive appearance (Figure 2A-2F). Some tumors were highly pigmented whereas others contained variable pigmentation or were amelanotic. Additionally, some mice had melanoma cells infiltrated in adjacent tissues as muscle, skin, and fat, making them difficult to separate for analysis. This phenomenon was most evident in the animals of G2 and G4, which presented mesentery metastasis.
Figure 2: Maleimides reduced the tumor size and the number of animals with tumors. (A; B) Images showing the macroscopic appearance of the tumor after twenty-one days of B16F10 cell subcutaneous injection in mouse treated with vehicle (G2). (C) Images showing the macroscopic tumor appearance from a mouse treated with (0.27 mg/kg) M2 (G4); (D) M7 (G8); (E) M5 (G6); (F) M9 (G10); (G) Images (400 x) of tumor sections stained with HE from a mouse treated with vehicle (G2); (H) M2 (G4) (Images 200 x). (I) Mean tumor size in mice after twenty-one days of subcutaneous injection of B16 F10 cells. (J) Percentage of animals that developed tumors after twenty-one days of subcutaneous injection of B16F10 cells. * p<0.05 compared to G2.
Different responses of malignant melanoma treated with maleimides derivatives were observed by in vivo assays. Comparing the effects of maleimides in vitro tests and in vivo tests, it was observed that while the IC50 for M5 and M7 were higher when compared with M2 and M9, they were more effective in vivo (Figure 2). The treatment with M5 (G6) significantly reduced the tumor size (3.73 ± 0.4 mm, 67% tumor size reduction), and only 37 ± 2% of mice developed a tumor. Likewise, the treatment with M7 (G8) inhibited 32% of the tumor growth (7.59 ± 0.61 mm), and only 42 ± 4% of mice developed a tumor. Briefly, tumor growth was significantly reduced by treatment with M2, M5 and M7 but not with M9 (Figure 2I), and the groups that received M5 and M7 showed significantly reduction in the Number of mice with tumors Figure 2.
The lungs (Figure 3E and 3F) and the mesentery (Figure 3G and 3H) from mice inoculated with B16F10 cells and treated with maleimides were examined postmortem. The macroscopic and microscopic pulmonary and mesentery nodules were analyzed and compared. Macroscopically visible, the tumor nodules showed black pigment spots of approximately 1-3 mm of diameter and tending to be fused.
Figure 3: Macroscopic and microscopic characteristics of lung and mesentery metastasis. (A; B) Images (400 x and 200 X, respectively) of mice lung sections stained with G2 and G6 HE. (C; D) Images (400 x) of mice mesentery sections stained with G2 and G4 HE. (E; G) Images showing the macroscopic characteristics of the metastatic nodules from lung and mesentery of the untreated mice (G2) and (F; H) the mice treated with M5 (G6). (I) Percentage of the pulmonary metastatic and hepatosplenomegaly. * p<0.05 compared to G2.
Secondary metastasis in the lungs and mesentery appeared more frequently in the positive control group (G2) (Figure 3A, 3C, 3E and 3I), conversely, the treatments with M5 (G6), and M7 (G8) drastically reduced lung metastasis (Figure 3B and 3F) and mesentery metastasis (Figure 3H).
The lungs of G2 animals showed evident tumor nodules, whose cells showed a prominent nucleolus and intracellular melanin deposition, and clear areas of necrosis were also detected. The treatment with M2 (G4) inhibited 10% of lung metastasis development, and several points of mesentery metastasis were only observed macroscopically with this treatment (Figure 3G), which was confirmed microscopically (Figure 3D). Among the animals treated with M5, only two showed lung metastasis microscopically, but not macroscopically. Only the treatment with M7 (G8) reversed the hepatosplenomegaly caused by the melanoma tumor Figure 3.
Biochemical and hematological analyses
To evaluate the toxicity produced by maleimides, the hematological profiles of the animals were determined and compared with the respective values obtained for the control group (G1). The results of the hematological parameters of the animals are shown in Table 2.
Hematological parameters show that the maleimides were not able to promote significant changes because hematological abnormalities occurred only in the groups with tumor (group +). The animals of the (+) groups showed significant changes in hemoglobin and leukocytes compared to (-) groups; these changes were caused by the tumor in development and not by maleimides themselves. The results from murine biochemical studies are summarized in Table 3.
Only the treatment with M9 showed possible hepatic toxicity. The enzyme activity values of ALT (165.3 IU/l) and AST (237 IU/l) were changed by treatment with M9 when compared with the activity values of the same enzymes for G1, which were 131 IU/l and 171 IU/l, respectively. M5 increased the values of ALT in relation to G2 (146.3 IU/l). Other parameters did not present significant alterations, which probably represent low toxicity of the maleimides.
Animal body weight changes
At the end of the experiment, the mice were weighed and the variation of body weight was evaluated. There was a statistically significant increase in the body weight of mice treated with M2 (G4). The weight of the animals treated with M7 (G8) increased by 2-3 g, but this positive weight variation was significantly different from those of G8, G1, and G2 mice, which gained more weight during the entire experiment Figure 4.
Oxidative stress in the liver after treatment with maleimides
The levels of thiobarbituric acid reactive substances (TBARS) increased in the livers of mice bearing treated and untreated melanoma tumors, and this effect was reverted only by treatment with M5 (G6) (Figure 5A). The mice treated with M7 (G8) and M9 (G10) showed significant increases in TBARS levels (20 and 15 nmol/g tissue, respectively) compared to G1 (2.81 nmol/g tissue). These maleimides seem to trigger an oxidative stress, which may be their mechanism of action as Antitumoral. Additionally, M9 also induced alterations in hepatic enzymes (ALT and AST), which is an indicative of hepatic toxicity.
However, the liver histology did not reveal any evidence of degenerative changes, steatosis or necrosis. Indeed, microscopic analysis of the selected organs did not reveal any treatment-related toxic effects. However, in some mice treated with M7 (G8) and all mice treated with M9 (G10), histological analyses showed megakaryocytic cells in the spleen.
The level of oxidative stress metabolites NPSH in liver were significantly reduced. The results are shown in the Figure 5B; animals treated with M5 (G6) (0.45 mm), M7 (G8) (0.21 mm), and M9 (G10) (0.24 mm) are compared with G1 and G2 animals (0.71 mm and 0.63 mm, respectively) Figure 5.
Imide derivatives are an important group of bioactive compounds showing androgen-receptor antagonistic, anti-inflammatory, anxiolytic, antiviral, antifungal, antibacterial, and antitumor properties, principally in the treatment of carcinoma [5,6,10]. The synthetic cyclic imides such as N-phenylmaleimide are easily obtained with high yields and present satisfactory pharmacological activity [11]. These compounds are hydrophobic and neutral and, therefore, able to cross biological membranes. Some pharmacological proprieties of N-phenylmaleimides are related to the size and electrophilic characteristics of the substituent groups on the imide ring and the aromatic ring, which can modify their steric properties or lipophilicity [5,6].
In the present work, the strain of murine melanoma B16-F10 was used; it is useful for in vitro melanoma model and is highly tumorigenic in vivo. This type of tumor grows spontaneously in mice and with great ability to develop metastases. The results presented in Table 1 indicate that the maleimides are capable of inducing cell death at low concentrations (between 18 and 11 μM), whereas the IC50 values after 48 h incubation were between 8 and 12 μM and, even lower after 72 h (between 8.5 and 4.5 μM). Among the imides M2 and M9, although presenting good cytotoxicity in vitro, in preclinical trials they presented different pattern of action. The imide M9 showed no ability to reduce tumor size (Figure 2), what could be result of a structural feature of M9, which contains one imídico ethyl group attached to the ring, while the M2 has no such group. In preclinical testing the effect of M2, M5 and M7 in the reduction of subcutaneous tumors were similar despite the structural difference between them. Therefore, it indicates that the presence of the -CH3 group, an electron donating group in the phenyl group does not affect the antitumor property of these imides. Similar results were obtained in studies carried out by Prado et al. [6], which showed a significant cytotoxic effect on this cell line of similar imides both, in vitro and in vivo.
It is worth noting that although the compound M9 is as cytotoxic as the others, it was not as effective in vivo. Whereas, several factors involving the pharmacodynamics and pharmacokinetics of a given compound may interfere with its performance in vivo, this fact should not be surprising, as also does not invalidate the results in vitro. The biotransformation of a drug often influences the magnitude of its effects in vivo as well as its absorption, distribution and excretion [17]. Thus, the fact that the imides studied have shown promising results in vitro, did not mean that they could exert such effects in vivo. However, when the compounds are also effective in vivo as it was shown in this work we might suggest some of these polyimides, including M5 and M7 may be promising candidates for anticancer drugs.
Mitochondria are indispensable for energy production and are crucial regulators of cell death, principally in the intrinsic pathway of apoptosis [18]. Alterations of mitochondrial membrane potentials were observed when B16F10 cells were treated with the N-phenylmaleimides, and the demonstrated uncoupler effect can be related to the mechanism of action of the cellular death induced by these compounds (Figure 1). Curiously, M9, which presented higher cytotoxicity in vitro, did not show antitumoral activity in the in vivo assay.
Prado et al. [6], also showed that some N-phenylmaleimides inhibit oxygen consumption in the presence of adenosine diphosphate (ADP), and this effect is related to inhibition of the respiratory chain enzymatic complexes. The change in the mitochondrial potential induced by the maleimides is a very interesting result because alterations of mitochondrial parameters may be related with apoptosis.
The increase in oxidative stress observed by the TBARS and NPSH assay shows that the melanoma tumor induces the production of reactive species and leads to a consequent increase in lipid peroxidation. Growing evidences suggest that cancer cells in general are under increased oxidative stress compared to normal cells, which includes enhanced reactive oxygen species (ROS), increased accumulation of ROS-mediated reaction products and over-expression of antioxidant enzymes [13].
The treatment with M7 strongly induced the lipid peroxidation, suggesting that this effect can be related to its mechanism of action. The increased cellular oxidant may be implicated in several signal transduction pathways that eventually lead to the enhanced susceptibility of tumor cells. In previous studies, our group has shown that cytoplasmic and mitochondrial GSH depletion and free radical generation in tumor cells may be implicated in a redox potential alteration inducing cell death by apoptosis, probably through cytochrome c release [19-21]. Compounds that induce the oxidative stress in melanoma cells may provide a candidate target for therapeutic purposes. ROS production and activation of caspase-9 and -3 occur later in the apoptotic process and are frequently reported as a consequence of the redox imbalance [22].
The induction of oxidative stress may be associated with activation of caspases and consequently apoptosis, since some studies report that azaindazolyl-Indolyl-maleimides are able to activate caspases 3 and 7 and to promote a decrease in Bcl-2 expression and an increase in Bax expression in several types of human cancer lines [8,23]. Wang et al. [24] reported that N-1- adamantyl maleimide promoted alterations in cellular adhesion molecules ICAM-1 and beta-1 integrin, this effect may be associated with the ability of maleimides M5 and M7 to reduce pulmonary and mesenteric metastasis (Figure.3B, 3F and 3H).
According to the results shown in this article, the treatments with M5 and M7 were more effective than the treatments with M2 and M9 in the model used in this study, because M5 and M9 were effective in the reduction of tumor size and metastatic processes. The differences in the maleimides mechanisms of action are probably due to difference in their chemical structures. M2 is an unsubstituted N-phenylmaleimide, M9 has an ethyl group between the imide ring and the aromatic ring, and M5 and M7 have substituent groups in the aromatic ring, with hydrophobic and electron-donor characteristics.
A similar effect was observed by Cashman et al. [25] with N-aryl maleimides inhibiting Bfl-1 expression, one anti-apoptotic of Bcl-2 family protein. An analysis of structure-activity relationship revealed that the substitution on the N-phenyl ring and variation of amines in maleimide ring system and, the necessity of a chloro substituent as well as a double bond in the maleimide ring are the main structural aspects for inhibition of Bfl-1 [25].
Previous studies with maleimides demonstrated strong fungicidal activities against standardized and clinical isolates of C. albicans and non-albicans Candida spp., and this activity is dependent on the chemical structure of each maleimide. The authors also presented a study of maleimides stability in aqueous medium and proved that these molecules are stable [11].
According to the structure of maleimides, the distance between the imide ring and the aromatic ring, varied by the introduction of different substituents, may be an important factor related to their activities. Some authors suggest that the distance between the imide ring and the aromatic ring is directly related to the antifungal and antibacterial activities and other biological effects of these molecules [6].
The antibacterial activity of maleimides reported by Cechinel-Filho et al. [26] indicates that the cyclic imide double bond is an important factor in the activity. Electron-donating and electron-withdrawing substituents in the aromatic ring of N-phenylmaleimides decrease the activity of these compounds, indicating the possibility of steric effects. The distance between the aromatic and the imide rings when separated by methylene groups does not affect the antibacterial activity. From our results, it appears that the aromatic ring must be connected directly to the imide ring, and the substituent might be bound to the aromatic ring, such as the methyl group present in M5, which confers more lipophilicity. Considering our promising results and importance of the physicochemical parameters for the studied activity, our research group has planned the continuation of these studies looking for the biological evaluation of other related compounds and application of distinct methods of structure activity relationships.
Furthermore, the maleimides that possess a type of cyclic α,β-unsaturated ketone have been extensively explored as antitumor drugs because this chemical group usually increases cytotoxicity, DNA binding and topoisomerase I inhibition and acts as possible thiol-alkylating agent of topoisomerase II [27].
Confirming this hypothesis, several reports state that maleimides interact preferably with the hydrophobic domains of enzymes based on the fact that the inactivation of sulfhydryl groups is greatly affected by the side-chain length of the derivatives [8,28]
Hematological parameters are a valuable tool for assessing injuries in the liver, spleen and bone marrow that are caused by certain substances, and serum aminotransferases activities are known as markers of hepatotoxicity. An increase in the activities of these enzymes indicates possible toxic hepatitis. The results of the present study reveal a marked elevation in AST and ALT activities in mice treated with M9. The maleimides caused few alterations in the hematological parameters, a very good indication against the acute adverse effects associated with usual chemotherapies.
Ordinarily, liver cell damage is characterized by a rise in plasma enzymes (AST, ALT, LDH, GGT, etc.). High concentrations of AST appear in a number of tissues (liver, kidneys, heart and pancreas). However, ALT is primarily localized in the cytosol of hepatocytes. Therefore, this enzyme is considered a good marker of hepatocellular damage [29]. Thus, increasing the hepatic enzymes mediated by M9 treatment may indicate possible hepatic toxicity of this compound, although microscopic examination of the liver showed no alterations.
Splenomegaly is a common pathological change in tumor-bearing states and may be related to the activation of humoral immunity [30]. The splenomegaly observed in melanoma-tumor-bearing mice was reversed only by M7 treatment. However, according to the literature, the splenomegaly in tumor-bearing mice might indicate enhanced Th2-related humoral immunity, and its reduction by treatment implicates in the down-regulation of the Th2 response no affecting the functions of the macrophages and/or natural killer cells [30].
In summary, we showed for the first time that the N-phenylmaleimide derivatives present relevant pharmacological properties in vivo, including antitumoral activity and apparently slight toxicity. Treatment with N-phenylmaleimides caused few alterations in the growth and body weight of mice. Important aspects related to the structure and the antitumor activity was also shown, although a non-classic SAR study has been performed. The antitumoral effects observed in the present study for N-phenylmaleimides indicate promising applications, though further studies are required to elucidate the mechanism of their antimelanoma activity and to define other more active compounds using especially M5 and M7 as model.