ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Mohamed Yassine SAYAH1, Rachida CHABIR2 , EL MADANI Nadia4, Youssef RODI EL KANDRI1, Fouad OUAZZANI CHAHDI1, Hanane TOUZANI1, Faouzi ERRACHIDI3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Moroccan Orange peels are a promising commercial source of pectin. Their massive industrialization generates a huge amount of solid waste. This work aims to develop a new valorizing reuse way of this solid waste by developing the extraction process of value-added products such as pectin from orange peels, which is the waste of orange juice processing industry. In this context, we evaluated the yield of pectin according to the state of the substrate and the acids used. Results obtained show that the peels which have undergone a Steam Distillation (SD) gives the highest yield of pectin (40% using sulfuric acid) compared to Fresh Peels (FP) (25.71% using citric acid).One part of the (SD) peels was dried outdoors and Non-Conditioned (SDNC), while the other was Conditioned (SDC) at 4°C. The highest yield of pectin for each one was respectively 40% using sulfuric acid and 34.81% using hydrochloric acid. The citric acid gives a satisfying yield in each peels stats, 38.52% using on (SDC), 31.88% (SDNC) and the lowest yield was 25.71% (FPC). The result of this work has emphasized that sweet orange peels are a good source of orange essential oil and pectin and has the potential to become an important raw material for food processing industries.
Keywords |
| Orange peels, acid hydrolysis, Citrus solid waste, valorization, pectin. |
I. INTRODUCTION |
| Annual world production of citrus exceeds 88 million tons [1]. Nearly the half of this production is destined to juice production, and the rest including the peel and other byproducts is considered solid waste citrus [2]. According to the Moroccan Ministry of Agriculture, the country produces 1.3 million tons each year [3]. This production generates huge quantities of solid citrus waste that may cause environmental problems, particularly water pollution, because of biomaterials in orange peels such as peel oil, pectin,and sugar that stimulate aerobic bacteria which degrade organic matters into carbon dioxide, nitrates, sulfates and phosphates in water which create eutrophication phenomena. Our research tries to find a possible way to solve this environmental problem by the valorization of this solid citrus waste by producing molecules of interest, such as pectin. |
| Pectin is mainly used as gelling and stabilizing agent and as a thickener in food systems such as jams and jellies. Pectin is alsoused in the pharmaceutical industry in cases of heart disease and gallstones [4]. Pectin is a polysaccharide substance present in the cell wall of higher plants. It is present in the form of a linear α-D-galacturonic linked by glycosidic bonds (1-4) acid polymer. This chain may beinterrupted by units of α-L-rhamnopyranosyl linked in (1-2) and bearing side chains mainly composed of galactose and arabinose residues [5]. Pectin is extracted by the action of a hot dilute acid solution. To improve this valorization of local citrus peels, our study tends to establish the link between the type of acid extraction and the substrate conditions (orange peels) to have a better pectin yield. |
II. . LITERATURE REVIEW |
| Many investigators have studied the influence of different parameters on the pectin extraction fromdifferent sources. Maria Helene and colleagues extracted pectin from apple pomace aiming at establishing the optimum conditions for acid extractionusing citric acid [6].UruthiraKalapathy and Proctor [7], extracted pectin from soy hull using hydrochloric acid and the highest yield obtained was 28%. LEVIGNE Sébastien; RALET Marie-Christine and THIBAULT Jean-Francois [8], used sugar beet as substrate for pectin extraction. In addition to other factors, they studied the effect of nitric and hydrochloric acid on pectin yield and they found that different kinds of pectins can be obtained with good yields at pH 1.Sohair E1-Nawawi andFadia R. Shehataexctracted pectin from Egyptian Orange Peels using hydrochloric acid was used asthe extracting agent, the optimum yield obtained was in the range from 21-30% [9]. However, there have been few carried out on pectin extraction from industrial orange waste. |
| Srivastava and Malviya extracted pectin from industrial waste of orange peels and the yield obtained was 18.69% using ctric acid [10]. A comparative study of pectin yield from orange peels using different acids was carried out by Shakila et al [11] and they found that during the drying process it wasobserved that there was a considerable difference in the pectin yield using different acids. Furthermore, using hydrochloric acid for all peels that were dried undersun give a good pectin yield.. In this work we evaluate the pectinyield variation according to different acids and especially the hydrodistillation of the substrate before the extraction of pectin. The preparation process of the raw material is explained below in: Materials and methods. |
III. MATERIALS AND METHODS |
| A. SAMPLES PREPARATION |
| In our study, we used two batches of ground orange peels. The first batch was directly frozen while the second has undergone a steam distillation in order to extract the essential oils (EO). After distillation, peels were recovered and separated into two groups. One was dried outdoors, while the other was frozen. To summarize the different stats of orange peels are: |
| Zest which undergoes Steam Distillation and was Conditioned (SDC) |
| Peel that has undergone Steam Distillation and which does Not been Conditioned (SDNC) |
| A Fresh Peels which been Conditioned (FPC) |
 |
| Each of these orange peels state undergoes an acid hydrolysis using four different Mineral (MA) and Organic acids (OA), which are sulfuric, hydrochloric, acetic, and citric acid. |
| B. PECTIN EXTRACTION |
| Extraction of pectin was done, in triplicate, according to the conditions described by [12]; [13] (0.05M, 80°C, 1 hour). We choose to test the effect of four acids at the same conditions; temperature, concentration and extraction time. We have selected a mineral acids (sulfuric acid, hydrochloric acid) and organic acids (acetic acid and citric acid). |
| After that orange peel undergoes an acid hydrolysis, following the conditions mentioned above, the solution is filtered and centrifuged (3000 g for 10 min). The slurry was filtered through fine cheesecloth to provide pectin extract. After cooling, the pectin was precipitated by ethanol 96% (v/2v; extract/ethanol), stirred slowly and stored in a refrigerator overnight in order to fully achieve the precipitation of pectin [14]; [15] The mono-phase system (concentrated extract) was then changed to a two-phase system (colloid + liquid). Pectin was dried at 40°C and the weight was monitoring until its stabilization. |
| C. STATISTICAL ANALYSIS |
| Variance analysis (ANOVA) was used to define the optimum conditions concerning the acid used for pectin extraction and the peels’ state. Data were analyzed by analysis of variance and means were separated by least significant difference when significant F (P<5%) values were observed. |
IV. RESULTS AND DISCUSSION |
| After acidified hot water extraction and further precipitation by ethyl alcohol the yield of pectin obtained depends on peels stats and acids used. Several acids can be utilized for the extraction of pectin. According to [16], the acids used for pectin extraction were cheap mineral acids, such as sulfuric, hydrochloric acid and organic ones like citric and acetic acid. Articles published recommend the use of hydrochloric [17]; [18];[19]. The highest pectin yields obtained from different orange peels stats peel with Hydrochloric, Sulfuric, Citric and Aceticyielded 35.34, 40, 38.52 and 10.19% respectively, the highest pectin yield being in case of sulfuric acid procedure. The extraction process, the variety, and the orange peels state can affect pectin yield and it depends on the type of raw material and methods of preliminary treatment. Effect of different acids on pectin extraction from (SDC) |
| A. EFFECT OF THE NATURE OF ACIDS ON PECTIN YIELD EXTRACTED FROM A STEAM DISTILLATED AND CONDITIONED ORANGE PEELS (SDC) |
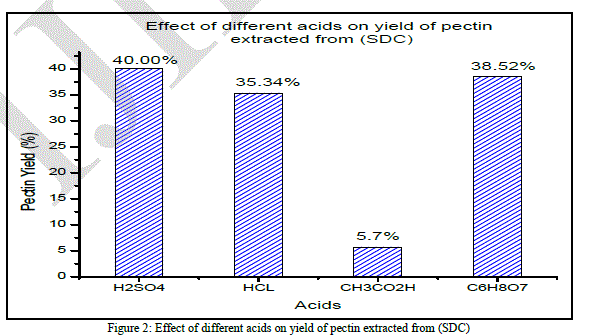 |
| According to (Fig. 2), the lowest yields were obtained when acetic acid was used (5.7% ± 0.0041).Although sulfuric acid showed the highest averageyield (40% ± 0.113), the variation was very large. While the average yield obtained using hydrochloric was (35.34% ± 0.0041). Citric acid had a good average value (38.52%±0.0059) and it is better than the other acid from an economic as well as from an environmental point of view. The analysis of variance indicated statistical significance in the yield. Peels state has a significant effect on pectin yield.To the mathematical interpretation, we should add an industrial vision which aims a law extraction cost and a better food tolerance. The ANOVA test shows that there is a significant difference between the different pectin yields mentioned above. The “F” (1312.959) value is very different from the critical value “F0” 4.06618557 and the probability is 4.1504E-11. These data confirm in a statistical way the experimental result. |
| In an economic vision we have collected some information. The following table regroups some observations targeting the volume reduction of citrus solid waste after it valorization by essential oil and pectin production. In this table we report the data concerning the highest pectin yield obtained using different acids on SDC. The yield of essential oil was 0.7%. |
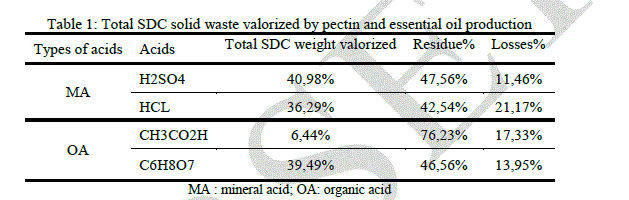 |
| Sulfuric, hydrochloric and citric acid give a satisfying volume reduction. The process involving pectin isolation by sulfuric hydrolysis after essential oil extraction, reduce the initial volume by nearly half, which is very interesting at the industrial scale. Pectin extraction by Hydrochloric acid reduces the initial weight after essential oil isolation by 36.29% and in the same conditions citric acid reduces the initial volume by 39.49%. The organic and non-corrosive nature of citric acid makes it suitable for pectin extraction. |
| B. EFFECT OF THE NATURE OF ACIDS ON PECTIN YIELD EXTRACTED FROM A STEAM DISTILLATED AND NONCONDITIONED ORANGE PEELS (SDNC) |
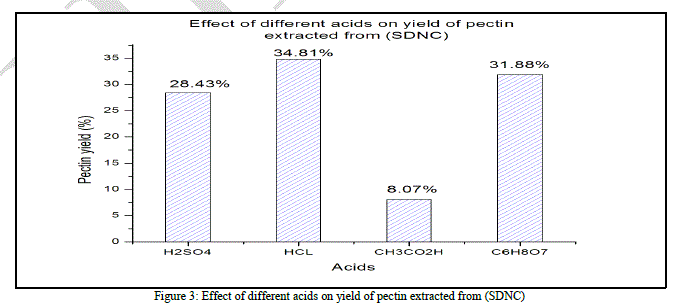 |
| According to (Fig. 3), and like the first case (SDC), the acetic acid provide the lowest yield of pectin (8.07% ± 0.005). Sulfuric acid is no longer the best acid in terms of yield (28.43% ± 0.015). the hydrochloric acid showed, in this case, the highest yield of pectin (34.81% ± 0.009) while the citric acid had a satisfying average yield (31.88% ± 0.013). The ANOVA show that the “F” value (351.482) is highly different from “F0” (4.06618557) and the probability is 7.8872E- 09. According to the ANOVA test, the difference between the four used acids is significant. |
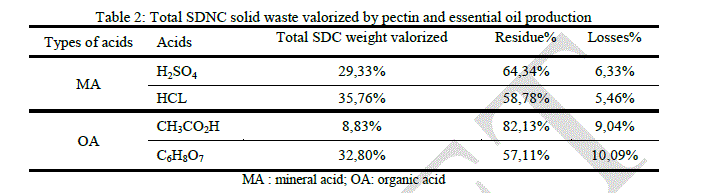 |
| Unlike the SDC case, this time, hydrochloric and citric acids give the highest Total SDNC weight valorization, 35.76% and 32.8% respectively. This result show that the acid hydrolysis efficacy is affected by the substrate pretreatment, it can be observed in the process involving sulfuric acid, only 29.33% of the total SDNC weight was valorized while it was 40.98% in the SDC case. |
| C. EFFECT OF THE NATURE OF ACIDS ON PECTIN YIELD EXTRACTED FROM A FRESH CONDITIONED ORANGE PEELS (FPC) |
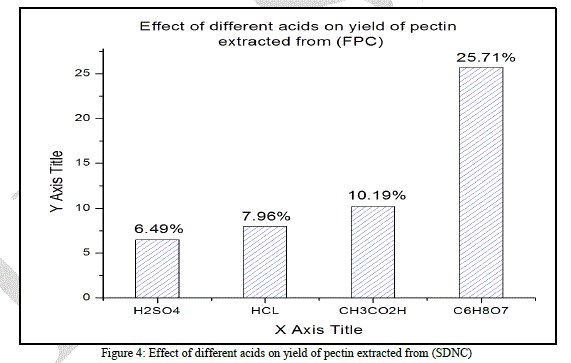 |
| As stated by the (Fig. 4) sulfuric, hydrochloric and acetic acids give a very low average yield of pectin, the average yields obtained are respectively 6.49% ±0.005, 7.96% ± 0.005, and 10.19% ± 0.006.In this case the citric acid gives the best average yield (25.71% ± 0.007). |
| SDNC gives the highest yield of pectin (max for sulfuric acid: 40% and min for 5.7% using acetic acid) followed by (SDC) that allows us to obtain an average yield around 34% using citric acid, and 8% as lowest yield of pectin (using acetic acid). The use of citric acid on (FPC) gave a maximum yield that does not exceed 26% while the yield obtained using others acids fall behind 10%. |
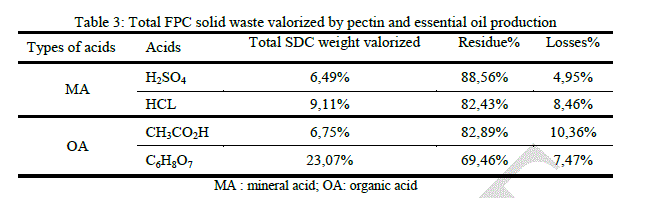 |
| Comparing to the two cases above (SDC and SDNC), total volume reduction in FPC case is too low, which is uninteresting. The highest yield obtained from the peels which have undergone a steam distillation can possibly be explained by the thermal effect of distillation that helps to release pectin from the peels. The difference in perceived yield between the frozen peels and those that are not (SDC and SDNC) can be explained by the effect of Freezing– thawing that can accelerate the release of pectin, producing de-esterification of pectin and soften the fruit tissue [20]. It has been established that the extraction of orange peels gives a high yield of highly esterified pectin with good gelling properties [21]. |
| Many studies report the effect of the acid used in pectin extraction. Shekharfound that the highest pectin yield obtained from orange peels residue left after simple distillation using citric acid was 46,46% [22]. After that the peel oil was removed, in our case, the pectin yield increased six times using sulfuric acid compared to peels that wasn't distilled, almost four times using hydrochloric acid.Contrary to mineral acids, organic ones have not shown a large difference in pectin yield according to peels stats. For “F. Kar” the pectin yield obtained with Hydrochloric acid was 29.58%, that was from orange peel and the oil was removed by petroleum ether before the pectin extraction [23]. Yeoh andKratchanova extracted pectin from fresh orange peels using hydrochloric acid. The yields obtained were respectively 5.27 and 18% [24];[25]. ShakilaBanu extracted pectin from sun dried orange peels using sulfuric and hydrochloric acid. The yields obtained were respectively 15 and 20% [11]. |
| D. COMPARISON OF AN EXPERIMENTAL OBSERVATIONS |
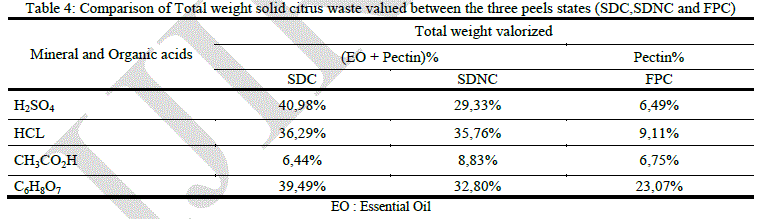 |
| Estimation of the volume reduction of solid orange waste is necessary and very important. It can give us an idea about the effectiveness of our valorization process. Table 1 report’s that the maximum volume reduction of solid waste is observed using sulfuric, acetic and citric acid hydrolysis on steam distillated orange peels. These acids give a good performance in terms of valorization on SDC, it is approximately 41% using sulfuric acid, 36.29% using hydrochloric one and 39.49% using citric acid. Totals valorized weights using these acids on SDNC are respectively 29.33%, 35.76% and 32.80%. Total weight valorized using fresh orange peels is very low. Acetic acid is the least important, in terms of valorization, among the selected acids. |
| The process involving organic acids gives us two different results. Citric acid allows us to have a satisfying volume reduction 39.49% which represents a significant benefit at the industrial scale, but the valorized volume of solid orange waste represent only 8.83% using acetic acid on SDNC. The uses of citric acid have many advantages, it is a noncorrosive and accepted organic acid in the food industry and furthermore it gives a good pectin yield. Moreover the process which involves the steam distillation of orange peels gives a higher pectin yield in addition to the essential oil it allows us to have a twice valorization of solid orange waste. The proper state for maximum pectin yield is "SDC", but a conditioned zest after hydrodistillation increases production cost. The shade dried zest (SDNC) generates no additional costs and allows us to have acceptable performance in pectin. |
V. CONCLUSION |
| Based on experimental observations, peels taken after the extraction of essential oil by simple distillation, give a higher pectin yield than the fresh peels. It is concluded that the process wherein the orange oil is first extracted by a simple steam distillation followed by acid extraction of pectin is more adequate for the industrial production of pectin. The previous results show also that the citric acid is adequate for the extraction of pectin because of its nature which is safe to food industry and the satisfying pectin yield it provides. The process of EO extraction then pectin isolation using citric acid hydrolysis technique is recommended. These results demonstrate the successful extraction of orange essential oil and pectin, with potential advantages for industrial extraction of pectin from an economic and environmental point of view. |
References |
|