ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Muppala Balaji1, Meena Vangalapati2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Nitrate salts are used widely in many areas as fertilizers in agriculture, explosives, oxidizing agents in the chemical industries, and as food preservatives especially to cure meats. If the range of nitrates increases in environment shows many dangerous and ill effects. Conversion of nitrate was needed. In this study TSS, TDS, BOD, COD and 93 % highest conversion of nitrate was found from the optimized conditions. The final form of the proposed model equation for the percentage conversion of nitrate was Ys=0.93(1-e-0.698t). Where Ys = percentage conversion of nitrate and t= conversion time (hr).The model showed good agreement with experimental data by generating average absolute relative deviation (AARD) of about 0.92± 2.9% conversion of nitrate from steel industry effluent using membrane separation process.
Keywords |
| Membrane separation, Modelling, average absolute relative deviation, steel industry effluent. |
INTRODUCTION |
Membrane technology: |
| Membrane separation processes operate without heating and therefore use less energy than conventional thermal separation processes such as distillation, sublimation and crystallization. Membrane separation process is widely used in the pharmaceutical industries, food technologies and biotechnologies |
| Reverse osmosis and electro dialysis are the important examples of membrane process. The TDS from waste water can be removed by reverse osmosis. Reverse osmosis is suitable for removing ions and larger species from industrial effluents with high efficiency (upto > 90%), clogging of the membrane by nitrates after long usage and high capital cost are the main drawbacks of this process. Reverse osmosis membrane process is suitable for removing high salt concentrations so that the treated effluent can be re-used again in the processing. |
| Nitrates[1]are naturally present in soil, water, and food. In the natural nitrogen cycle, bacteria convert nitrogen to nitrate, which is taken up by plants and incorporated into tissues. Animals that eat plants use the nitrate to produce proteins. Nitrate is returned to the environment in animal feces, as well as through microbial degradation of plants and animals after they die. By the aerobic action of the nitrosomonas bacteria [2] in nitrogen cycle the nitrates converts to nitrogen. Nitrate salts are used widely as inorganic fertilizers [3], explosives, oxidizing agents in the chemical industries, and as food preservatives especially to cure meats. Natural process the is no contamination of nitrate by the extensive utilization of synthetic fertilizers and Industries, human excreta, sewage disposal, cattle seepage, fertilizer industries, explosives industries, municipal waste and industrial effluents, particularly from food processing, release of improperly treated wastewater from industrial or municipal facilities are the causes of nitrate contamination in natural water, ground water systems as well as atmosphere also. Nitrate contamination causes health hazards[4] like methemoglobinemia which losses the oxygen carrying capacity of hemoglobin, blue baby syndrome, headache, dizziness, vomiting, diarrhea, labored breathing Pregnant women are more sensitive to the effects of nitrate due to a natural increase in methemoglobin levels in blood during the later stage of pregnancy beginning around the 30th week. Nitrates are entering into the atmosphere and can be converted to nitrous oxide (N2O), which a greenhouse gas is contributing to global warming, acidic deposition and the formation of other secondary pollutants. Nitrate is one of the main contributors to eutrophication[5] of surface water. The U.S. Environmental Protection Agency[6] (EPA) sets Maximum Contaminant Levels (MCLs) for nitrogen in public drinking water systems as 10 milligrams per liter (NO3 – N mg/l) and nitrites as one milligram per liter (NO2 –N mg/l). The World Health Organization (WHO) [7] has prescribed the maximum permissible limit of nitrate in drinking water as 50 mg per liter, while IS-10500 prescribes 45 mg per liter as the maximum permissible limit in drinking water. |
| In this study, Membrane technology has been adopted for the treatment of the steel industry effluent with the help low cost ceramic membranes .Ceramic membranes were out of clay which in turn very inexpensive when comparing with the other polymeric membranes. |
| The fabricated Membrane was arranged in an apparatus which consists of two tanks namely Clarification tank and Collection tank were shown clearly. The percentage removal of the selected nitrates from the industrial effluent was done by the process. The parameters like pH, Contact time, concentration of nitrates, TSS, TDS, BOD and COD were calculated. |
II. MATERIALS AND METHODS |
| Conversion of nitrate was more important to remove contamination in steel industry effluent contain more amount of nitrates. This effluent was collected from steel industry. To convert nitrates into nitrogen using membrane separation process. Finally for every one hr sample was collected from membrane and nitrates were estimated as shown in figure1. |
Treatment Technique – Membrane Technology: |
| Membrane Technology was adopted as one of the technique for the removal of nitrates from the steel Industry Effluent. Fabrication of membrane was done with Kaolin clay and other constituents, which were mentioned in table 1. Fabricated membranes were dried for a period of 24 hours and were kept in Muffle furnace at 900 for six hours and cooled for 15 hours in the muffle furnace. The membrane was kept for water washings repeatedly for the removal of ash content on the membranes. |
 |
Estimation of nitrate concentration |
| The concentration of nitrate was estimated by using spectrophotometer [8]. The reagents used for this process are salfanilic acid, hydrochloric acid, methyl anthranilate and sodium hydroxide. Take 10 ml of effluent and add 1 ml of salfanilic acid to diazotized to form nitrates. Then 1ml of 2mol/ l HCl was added to make the reaction faster and the contents are kept under shaking for 5 min to complete the diazotization [9] reaction. After the diazotization 1 ml of 0.5% methyl anthranilate was added to indicate the color. Methyl anthranilate reacts with nitrates present in sample and forms brown red color. For this 2 ml of 2mol/L NaOH was added to neutralize the acidic nature and 10 ml of distilled water was added for this, and the color of the sample was estimated under 490 nm spectrophotometrically to get the concentration of nitrate. |
Modelling of conversion of nitrate |
| In order to describe the nitrate conversion from sewage water the following hypothesis were used. The mass transfer coefficient is constant. The conversion of nitrate diffusion phenomenon by biomass derived from rotten fruits under aerobic conditions. The final form of modeling equation [10] was obtained from the conversion of nitrate |
| Ys= B (1-e-Dt) |
| Where |
| Ys = percentage conversion of nitrate. |
| T= conversion time (hr) and |
| B and D are equation constants. |
III. RESULTS AND DISCUSSION |
Percentage conversion of nitrate |
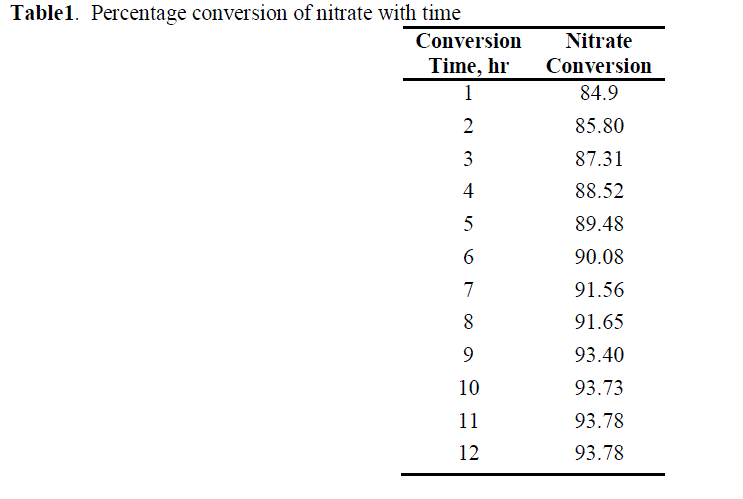
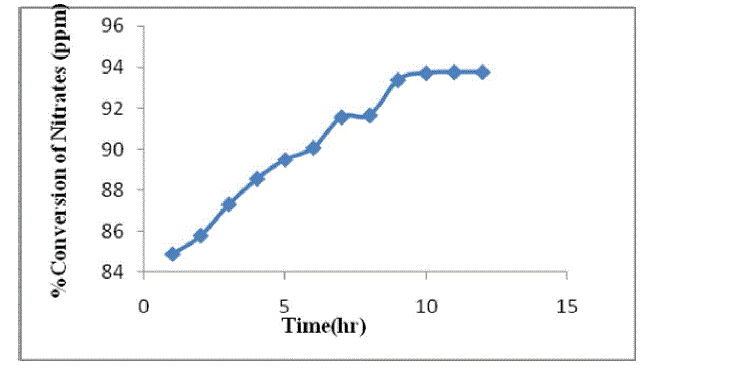
| As the reaction proceed ,denitrifying the effluent and converts the nitrates into nitrogen and releases to atmosphere. The concentration of nitrates is decrease as the time proceeds and the conversion increases[11]. From the optimized conditions the percentage conversion is found to be 93% and it remains constant from 9hr to 12 hr. The results are shown in table 1 and figure 2. |
| Table1. Percentage conversion of nitrate with time |
 |
| Fig.2. Percentage conversion of nitrate |
 |
 |
Modelling of conversion of nitrate by using membrane: |
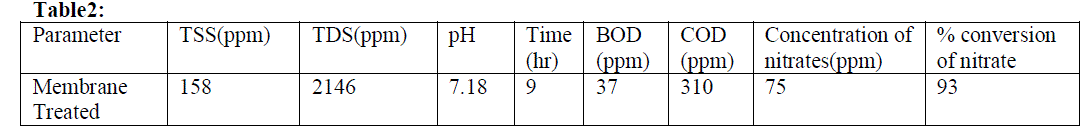
| To describe the conversion of nitrate in effluent using membrane. The highest percentage conversion of nitrate was found to be 93 % from the optimized conditions table 2. The final form of the proposed model equation for the percentage conversion of nitrate was[12] |
| Ys=0.93(1-e-0.698t). |
| Where |
| Ys = percentage conversion of nitrate |
| t= conversion time (hr) and |
| The model showed good agreement with experimental data by generating average absolute relative deviation (AARD) |
| of about 0.94± 2.9% conversion of nitrate from steel industry effluent using membrane separation process. |
IV. CONCLUSION |
| The highest percentage conversion of nitrate was found to be 93 % from the optimized conditions. The final form of the proposed model equation for the percentage conversion of nitrate was Ys=0.93(1-e-0.698t). Where Ys = percentage conversion of nitrate and t= conversion time (hr).The model showed good agreement with experimental data by generating average absolute relative deviation (AARD) of about 0.94± 2.9% conversion of nitrate from steel industry effluent using membrane separation process |
References |
|