ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Dr. K. Vijaya Kumar, Dr. G. Sunita Sundari & N. Krishna Jyothi Dept. Of Physics, K L University, Guntur-522 502(A.P)-INDIA |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
An attempt has been made in the present work to develop a new Polyethylene oxide (PEO) based Solid polymer electrolyte membrane, (PEO+KHCO3) by Isothermal immersion- technique. Several experimental techniques such as IR, composition dependence conductivity, temperature dependence conductivity in the temperature range of 308- 368K and transport number measurements were employed to characterize this polymer electrolyte membrane system. The IR studies confirm the salvation of K+ ion with PEO. The conductivity of the (PEO+KHCO3) electrolyte was found to be about 3 times larger than that of pure PEO at room temperature. The transference data indicated that the charge transport in these polymer electrolyte systems is predominantly due to ions. Using this conducting polymer electrolyte membrane, a polymer electrolyte membrane (PEM) Fuel cell stack was fabricated with the configuration anode/polymer electrolyte/cathode and studied its I-V Characteristics. The results of these investigations are reported in this present paper.
Keywords |
| Isothermal immersion, polymer electrolyte, ionic conductivity, transport number, Polymer Electrolyte Membrane(PEM)fuelcell,current(I)-voltage(V)Characteristics. |
INTRODUCTION |
| The two primary concerns of scientists today are energy and environmental with the rapid depletion of fossil fuels. There is an urgent need to look for clean alternate source of energy. Fuel cells satisfy both these criteria. It’s high time that we realize the need for cutting edge technologies which satisfy both the conditions i.e. being able to match the present day demand and also being reliable. One such convincing technology is “Fuel cell technology”. The necessity for this technology has been widely accepted globally in the energy and power sectors. Of the many emerging technologies such as Biomass Fuels, liquid-bio fuels, geothermal energy, Photo-voltaic, Hydro-electric power, solar thermal-electricity, tidal-energy, wind-energy, fuel cell technology has been much considered comparative with other technologies and if developed, has more advantages because it has the practicability of being used in many mobile applications and hence this seems to be the futuristic technology [1-2]. |
| On the world wide automobile market technical developments are increasingly determined by the dramatic restriction on emissions and increasing pollution levels. Therefore there is a necessity for a completely new technology to break through if it offers an answer by its principle, meeting the requirements of resource preservation and emission restrictions. The fuel cell technology has the potential, in terms of environmental compatibility and efficiency [3] .Fuel cells are an important technology for a potentially wide variety and range of applications including transportation power for automobiles, stationary and distributed power for rural areas, portable power for laptops, mobile phones etc. it will be decided by the costs of fuel cell systems if they will be used as power generators in future. Presently the objective is to lower the costs for all types of fuel cells by mass production. |
| The research and development of polymer electrolytes for ambient temperature rechargeable lithium and solid ion batteries has always an Identifiable. The ionic conductivity in high molecular weight polyethylene oxide (PEO) doped with sodium and potassium salts were first reported by P.V.Wright [4]. The technological implications of these PEO based electrolytes, such as their use in lithium batteries were realized and suggested by M.B.Armand [5]. These reports led to significant research activities around the world in the last 20 years. Exploratory investigations, prototype developments and fundamental issues related to these solid electrolytes have been reported in reviews by many researchers [6, 7]. |
| In general, the polymer component of PEO based composite electrolyte is a mixture of crystalline and amorphous phases, ratio of which depends to a great extent on compositions of the electrolyte, temperature and thermal history. The solid polymer electrolytes i.e. polymer salt complexes are of technological interest due to their possible application as solid electrolytes in different devices such as energy conversion units (batteries/fuel cells), electro chromic display devices/smart windows and photo-electrochemical solar cells etc [8-11] Subsequently studies on PEO-based polymer electrolyte complexes using alkali salts [11-13] were developed in this field. |
| In the present paper, the authors report solid ion conducting polymer electrolyte based on (PEO+KHCO3) membrane system. Several analytical techniques, such as IR, composition dependent conductivity, transport number measurements and temperature dependent conductivity in the temperature range 308-368K were performed to characterize these polymer electrolytes. Based on these solid conducting electrolytes, a PEM Fuel cell stack was fabricated with the configuration anode/polymer electrolyte/cathode and studied its I-V Characteristics. The results of these investigations are reported in this present paper. |
EXPERIMENTAL |
| Pure PEO (Aldrich = 6x105) complexed with a fine powder of potassium bicarbonate salts (KHCO3) were prepared in the stoichiometric ratios (90:10), (80:20), (70:30) by an Isothermal immersion- technique using methanol (water free) as solvent. The IR spectra in these films were recorded with the help of JASCO FT/IR-5300 spectrophotometer in the range 400-4000cm-1. |
| The dc conductivity has been measured using the lab made conductivity set up [13] in the temperature range 303-368K. The ionic and electronic transport numbers (tion and tele) were evaluated by means of Wagner’s polarization technique [14, 15]. In this technique, a freshly prepared film of PEO+KHCO3 under a dc bias (step potential 1.5V) was used. The resulting current was monitored as a function of time with a keithley electrometer (Keithley Inc., model 614). |
| Finally a PEM fuel cell was fabricated with the configuration anode/polymer electrolyte/cathode and studied its I-V Characteristics. The details about the fabrication of Polymer electrolyte fuel cells are given elsewhere [16, 17]. The current-voltage characteristic studies of these PEM fuel cells stack were monitored at a constant load of 0.5Ω. |
RESULTS AND DISCUSSION |
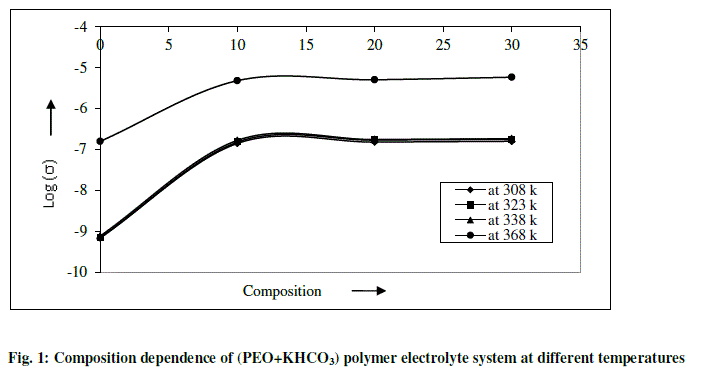
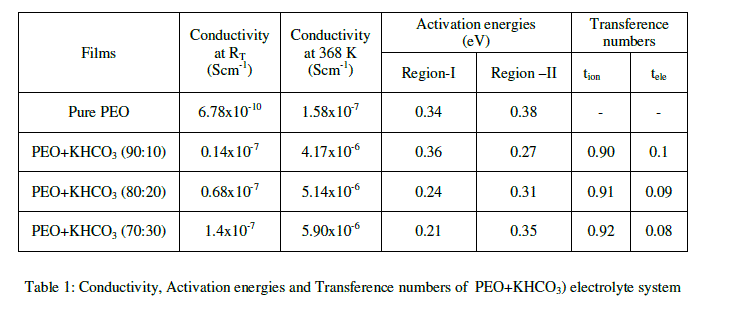
| The variation in dc conductivity (σ) as a function of KHCO3 composition in PEO from room temperature (RT) to 368K is given in Fig 1. Conductivity data at RT and 368K are reported in Table 1. The following conclusions can be drawn. |
| (a) The conductivity of pure PEO is approximately 10-10 Scm-1 at room temperature and its value increases sharply to 10-7 Scm-1 on complexing with 10 wt % KHCO3. The increase in conductivity becomes slower on further addition of KHCO3 to the polymer. This behavior has been explained by various researchers, who have studied PVP and PEO based polymer electrolyte in terms of ion association and the formation of charge multipliers [18-21, 23] |
| (b) The ionic conductivity in the polymer complexes may be interpreted on the basis of a hoping mechanism between coordinating sites, local structural relaxations and segmented motions of the polymer chains. These are essential to assure high conductivity of the electrolyte [22-24]. |
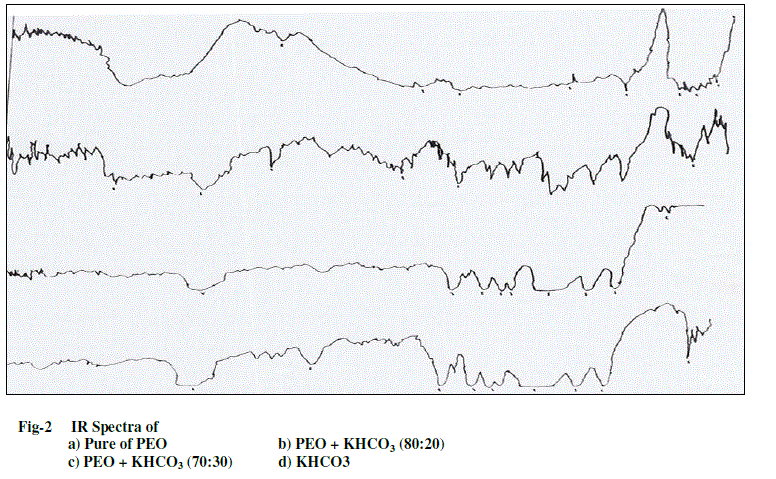
| The complexed polymer PEO and salt KHCO3 were confirmed by using IR spectroscopy. The IR spectra of pure PEO, PEO complexed with KHCO3 were recorded with the help of JASCO FT/IR–5300 spectrophotometer in the range 400- 4000 cm-1 and are shown in Fig-2. The intensity of the aliphatic C-H stretching vibrations band observed around 2950cm-1 in PEO was found to decrease with the increase in the concentration of KHCO3 salt in the polymer. The width of the C-O stretching band observed at around 1100cm-1 in PEO also showed an increase in the salt concentration in the polymer. Also the appearance of new peaks along with changes in existing peaks (and/or their disappearance) in the IR-spectra directly indicates the complexation of PEO with KHCO3. This IR data clearly establishes the complexation of KHCO3 with different weight ratios of the polymer PEO. |
 |
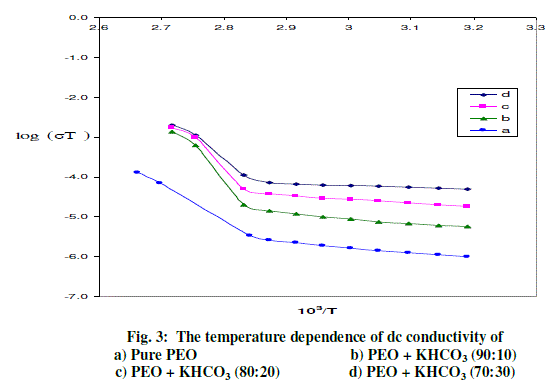
| The variation in conductivity as a function of temperature for pure PEO with different compositions of (PEO + KHCO3) polymer electrolytes over the temperature range of 303-368 K is shown in Fig-3. The conductivity increases with temperature in pure PEO and also in all the compositions of polymer electrolyte systems. The conductivity versus temperature (log σT versus 103/T) plots follows the Arrhenius nature throughout, but with two different activation energies above and below melting point (Tm) of the polymer. In region- I (i.e., below Tm) the conductivity of pure PEO increases slowly with temperature up to 65oC. At 65oC there is a sudden increase in conductivity. In region- II (i.e., above Tm), the conductivity again increases with temperature. The calculated conductivity (σ) at room temperature, at 368K and activation energies (Ea) for pure PEO and (PEO+KHCO3) electrolyte systems are given in Table 1. The conductivity of pure PEO is = 6.78*10-10 Scm-1 on complexing with 10 wt % KHCO3. Further addition of KHCO3 to the polymer, the increase in conductivity becomes slow. This behavior has been explained by various researchers, who have studied PEO and PEO based electrolytes, in terms of ion association and the formation of charge multipliers [25- 29, 32]. |
 |
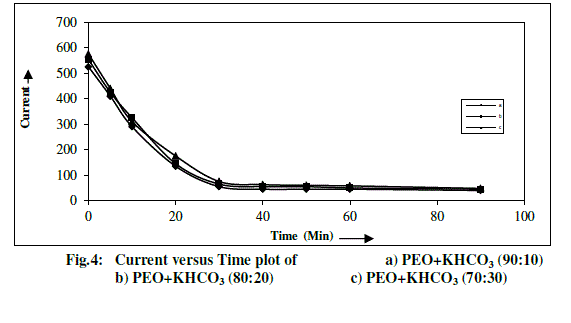
| Total ionic transference number of polymer electrolyte has been measured by Wagner’s Polarization technique [14, 18], which is used to determine the ionic contribution to the total charge transport by measuring the residual electronic current passing through the electrolytes. Three Wagner polarization cells Ag/ electrolyte/Ag were prepared by coating silver (Ag) paste as blocking electrode on to the faces of PEO+KHCO3 based composition polymer electrolyte systems. A fixed small dc potential (1.5V) has been applied across the blocking electrodes and the current passing through the cells is measured as a function of time for one hour to allow the samples to become fully polarized are shown in Fig.4. |
 |
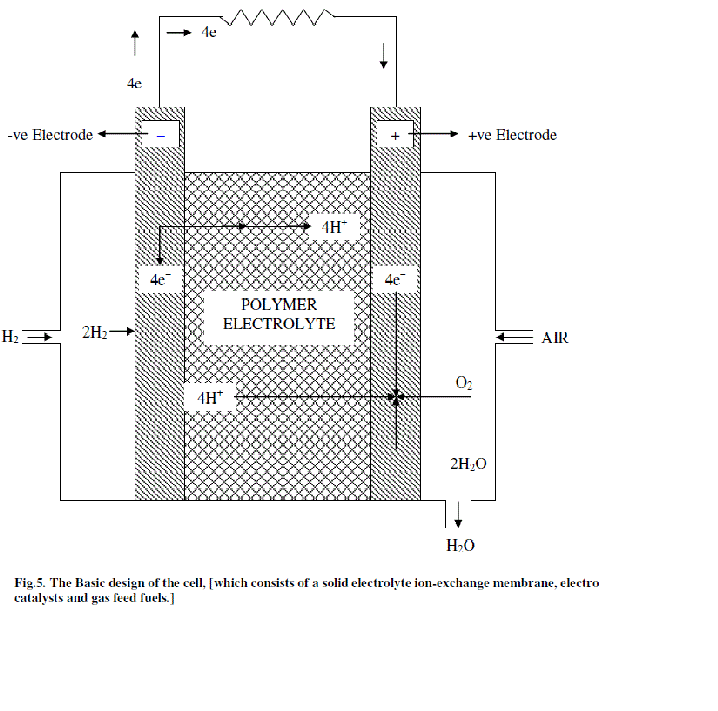
| The basic design of the cell, which consists of a solid electrolyte ion-exchange membrane, electro catalysts and gas feed fuels is illustrated in Fig.5.The distinctive feature of this cell is that it uses a solid polymer electrolyte in the form of an ion-exchange membrane. The membrane is non-permeable to the reactant gases, hydrogen and oxygen, which thus prevents them from coming into contact. The membrane is however; permeable to hydrogen ions [33] which are the current carriers in the electrolyte .The desired properties of polymer electrolyte membrane are high ionic conductivity, low permeability of fuel and oxidant, Very low electronic conductivity, high resistance to its oxidation or hydrolysis and Mechanical stability [34]. |
| The two electrodes, which consist of the electro catalyst and a plastic material for water-proofing the electrode, are in the form of fine metallic wire screens. They are bonded on either side of the electrolyte layer. The wire screen material is titanium or platinum. Metallic current collectors are ribbed onto each electrode. The hydrogen compartment of the cell is enclosed; the hydrogen gas enters this compartment through a small inlet and circulates throughout the ribbed current collectors and distributes itself evenly over the electrode. On the opposite side oxygen or air enters the compartment, coolant tubes run through the ribs of the current collectors. On the oxygen side, the current collectors also hold wicks which absorb water, the product of fuel-cell reaction and carry it over by capillary action. The water leaves the cell through an exit from the oxygen compartment. Oxygen is prevented from leaving its compartment by the inclusion of a differential pressure water-separation system. |
| The ion-exchange membrane electrolyte is acidic and the current carriers in solution are hydrogen ions. The hydrogen ions are produced by the reaction at anode according to, |
 |
| The single cell1 with the configuration Anode / (PEO+KHCO3) (90:10)/ Cathode was assembled with the specifications and the first readings were taken with the cylinder gases. The specifications of the single fuel cell with the polymer electrolytes of different wt% of (PEO+KHCO3) (90:10), (PEO+KHCO3) (80:20) and (PEO+KHCO3) (70:30) were given in Table-2 respectively. The resistor 0.5 were added in series to the circuit to gain an increase in voltage. Subsequently it was achieved. The open circuit voltage was found to be 0.95 volt. And also studied its I-V characteristics. |
 |
| Table-3: Specifications for the THREE PEM Fuel cells stack |
| For the three cell stack we used non-porous, bi-porous graphite plates. The three design aspects of the PEM fuel cell system under fabrication are |
| (a) The hydrogen on demand generation system (i.e. hydrogen reformer system) |
| (b) The air scrubber system (i.e. the fuel cell assembly) |
| The incorporation of the above three designs completes the fuel cell system [35]. Using these conducting polymer electrolyte membrane systems, a three fuel cell stack have been fabricated and the results are summarized below. |
| The area and thickness of the electrolyte are respectively 1.33cm2 and 150m. The I-V characteristics are studied for all the cells at room temperature with a constant load of 0.5 .were shown in Fig-6. It is observed that the open circuit voltage was found to be in range 0.95 V to 1.1 V it may be due to used electrolyte membrane type and electro catalysts. |
 |
CONCLUSIONS |
| i. The conductivity of (PEO + KHCO3) (70:30) at RT was determined to be 1.4x10-7 Scm-1 and at 368K was 9x10-6 Scm-1. The charge transport in this polymer electrolyte (PEO+KHCO3) was predominantly due to ions. The fuel cell stack is found to be better performance as compared to a single cell fuel cell. |
| ii. [PEO+NaHCO3] three cell stack wattage is found to be 4.25 watt with a stack voltage of 0.85 volt. |
| iii. The fuel cell characteristics like I-V characteristics, efficiency, power density etc. mainly depends on nature of electrolyte, the generated gases and its purity. But it is observed that the volt-ampere characteristics, efficiency of these fuel cells are very low. Further work has to be done to improve the fuel cell efficiency by proper choosing of solid polymer electrolytes along with the electrodes. |
| iv. The improve power density current density; efficiency of the fuel cell mainly depends up on the nature of catalyst used in the fabrication. Among all the electrodes, platinum is a very good catalyst but, its cost is very high. Therefore studies of other catalyst materials in the place of platinum are needed. |
ACKNOWLEDGEMENTS |
| The authors thank Er. Koneru Satyanrayana, President, K.L.University Guntur (A.P) for his encouragement and support and also the author highly grateful to Dr. R.Sreehari Rao,Vice Chancellor K.L.University. Finally the author thanks Prof. A.Ananda Kumar Principal, KLU College of Engineering and Dr. K. Rama Krishna, Dean Academics Prof. S. Venkateswarlu, Coordinator, FED K.L.University for his constant support. |
References |
|