ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
P. BalaRamesh 1, P. Venkatesh 2, A. Abdul Jabbar 3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This article focuses on the effects and applications of ethylenediamine dithiocarbamate (EDADTC) on coordination and electro chemistry. EDADTC shows stable palladium (II) metal complex in organosulphur chemistry. Since they have soft sulphur donor atoms can make a strong metal binding property, act as the ligand and stable above pH 12. Due to their water soluble tendency, flow of electron from alkyl group to nitrogen, inductive effect and conjugation effect proved the stabilizing property in electroless plating process. EDADTC has improved the physical property and produce the quality deposits in electroless copper plating process. The palladium (II) metal complex was characterized by Rapid Elemental Analysis. The structure of the stable complex was confirmed with Electronic and I.R Spectral Analysis. The surface morphologies of copper deposits were characterized by AFM studies. The crystallite sizes of the copper deposits were measured by XRD studies and the quality of the copper deposits was investigated by cyclic voltammetry studies
KEYWORDS |
| conjugation effect, copper deposits, crystallite sites, ligand, surface morphology |
I . INTRODUCTION |
| The importance of co-ordination chemistry has now become broad and the applications of co-ordination compounds to the scientific world are tremendous.[1] Several donor atoms like oxygen, sulphur, nitrogen, phosphorous were reported earlier by many pioneer workers in this field. Dithiocarbamates have been invented early in the history of organosulphur chemistry. Synthesis of dithiocarbamic acid was first reported by Debus [2] in 1850. Delpine and many workers had taken venture in developing dithiocarbamates and several metal were synthesized due to the versatile nature of dithiocarbamates. Dithiocarbamates and dithiophosphates are the most widely studied complexes today. Because of the metal biding property and their chelates vital role to biological, agricultural and industrial field etc. |
| Dithiocarbanmate complex has a lone pair of electron on the nitrogen astom, that becomes more important for the donation of the electron for higher oxidation states of the metal. The π electron flow from the nitrogen atom to the sulphur atom via., a planar delocalized π orbital system is the special feature of π dithiocarbamates. The net effect is a strong donation, resulting in a high electron density on the metal. |
| In 1956, Chatt and co-workers reported a detailed electronic and IR studies of a number of dithiocarbamate complexes and concluded that the resonance form. In the field of rubber industry, dithiocarbamate are used as vulcanization accelarators, antioxidants, and floatation agents. [3,4] In the field of medicine, dithiocarbamates are used as antifungal, antiviral, anticancer drugs. They are used in the control of various dermatopytes. The used of tetraethylthiuram disulfide for treatment of scabies was reported by Jordan in 1942 and their intermediates have been founds useful in the treatment of chronic alcoholism.[5] |
| Alkyl derivatives of dithiocarbamates complexes have many commercial application and are successfully used as fungicides and pesticides. [6] In the field of agriculture, tetraethyl thiuramdisulfide and dimethylamine dithiocarbamates are used for vegetable and corn seed treatment and extensively used for the control of apple scab is ferbam, the iron salt of dimethyl dithiocarbamates. Red stele decreases of strawberry was controlled by disodium ethylene dithiocarbamates. The important use is to cause a zone of act inhibition opposition to the bacterial cell. |
| Electroless (or) autocatalytic copper plating step is most important for Through Hole Plating (THP) of Printed Circuit Boards (PCBs). Various complexing agent have been used in electroless copper bath namely xylitol, mannitol and Dsorbitol were reported as environmentally friendly alternate copper (II) ligand in solution [7]. Formaldehyde is the most commonly used reducing agent [8] and play vital role in kinetics. [9-17] EDTA was used as most common complexing agent in electroless copper plating. [18-21] On the other hand EDTA was very weakly biodegradable. [22] Methane sulphonic acid (MSA) has gained more popularity in electroless copper plating related to electronic industries. Because methane sulphonate is characterized by excellent metal salt solubility, conductivity, bath stability and bio-degradability. Additions of methane sulphonic acid (MSA) in a small volume produce uniform and high quality coating.[23] |
II . RELATED WORK |
| The complex of Palladium metal ion with ethylenediamine dithiocarbamate is found to be unreported in the literature survey. Since the complex have water soluble due to their hydrophilic moiety, used as crown of chelate, biological activities, agricultural and widespread industrial applications, this investigation wil pave a way for new finding. Many stabilizers has been reported in electroless copper deposition process. But the use of substituted dithiocarbamate as stabilizer in electroless copper plating process work is unreported. The influence of ethylenediamine dithiocarbamate on Palladium (II) complex and act as stabilizer on new bath based copper methane sulphonate replacing copper sulphate. Saccharose and xylitol were used as complexing agents with para formaldehyde has been developed the bath. |
III. EXPERIMENTAL |
Equipments |
| The molecular weights, of the complexes synthesized were determined using the Beckmann freezing point apparatus. The molecular formula of the complexes was ascertained using elemental analysis and molecular weights. Haraeus-Chn-O-Rapid analyser (West Germany) was used for the estimation of carbon, hydrogen, sulphur and nitrogen contents present in the complexes. Electronic spectra data were obtained with (Hitachi 210 modal) using a regular quarts cuvette 10 mm path length. Infrared spectra were recorded on a (Hitachi 210 modal) 200-4000 cm-1 using KB pellet. |
| The crystal structure of the copper deposits was investigated using XRD peaks and roughness of the deposits was characterized by AFM studies. Cyclic voltammetric curves were obtained by standard electrochemical analyser CHI 600D Austin USA. |
Reagents |
| 1. Ethylenediamine (Qualigens) |
| 2. Ammonia solution (Fisher) |
| 3. Carbon- disulphide (S.D. Fine Chem. Ltd) |
| 4. Absolute ethanol (Reid-de-Ham) |
| 5. Dry ether (Qualigens) |
| 6. Saccharose (Fisher) |
| 7. Xylitol (Qualigens) |
| 8. Copper as methane sulphonate |
| 9. p-formaldehyde (Fisher) |
| 10. KOH (Fisher) |
Preparation of stock solutions |
| The 0.1 m stock solutions, Palladium (II) chloride were prepared by dissolving the required quantities of metal salts in H2O:H2SO (25:75). About 50g of copper carbonate was weighed and transferred into 500ml clean beaker and treated with approximately 60ml of methane sulphonic acid till the evaluation of carbon-di-oxide gas. Minimum quantity of double distilled water was added and the solution was made up to 250 ml standard measuring flask. The oil and suspended impurities present in the solution were removed by filtration and stored in a clean container. One ml of stock solution was analyzed by using N/10 of standard sodium thio sulphate and calculated the amount of copper. |
Preparation of Ehtylenediamine Dithiocarbamate |
| M. M. Jones et al [24] reported the synthesis of Ethylenediamine Dithiocarbamate in 1979. 45 ml of concentrated aqueous ammonia (28%) 20ml of Ethylene diamine (0.33 mole) and 42 ml of absolute ethanol were added to a beaker equipped with a magnetic stirrer. A mixture of 81 ml of carbon disulphide dissolved in 45 ml ethanol was added in drop wise to the reaction mixture with constant stirring such that the temperature did not rise above 48°C, while adding the mixture. The clear yellow reaction mixtures become turbid upon addition of about 23 ml of a mixture containing carbon disulphide and ethanol solution. The off white precipitate settled shortly after the addition was complete and the reaction mixture was treated with 150 ml of anhydrous ether and stirred for 1.5 hrs. The beaker was covered with a film and allowed to stand overnight. The crystal formed were collected on a filter paper and allowed to air dry. |
Preparation of Pd (II) Ethylenediamine dithiocarbamate |
| Pd(II) (edadtc)2 chelate was prepared by mixing an aqueous solution of palladium (II) chloride and EDADTC in the ratio 1:2 metal to ligand. Palladium (II) chloride was added drop wise to EDADTC solution and warmed with constant stirring. The pH of the above reaction was maintained at 3-5, since the yield was found to be more at this pH. The reaction resulted in the formation of a light green precipitate. It was filtered and dried. |
Copper surface formation |
| The plating experiments were performed on pure copper sheet (2.0 X 2.0 X 0.1 cm) in a 100 ml glass beaker. The copper plate was polished with fine grid paper, rinsed with double distilled water and carried out by following procedure. |
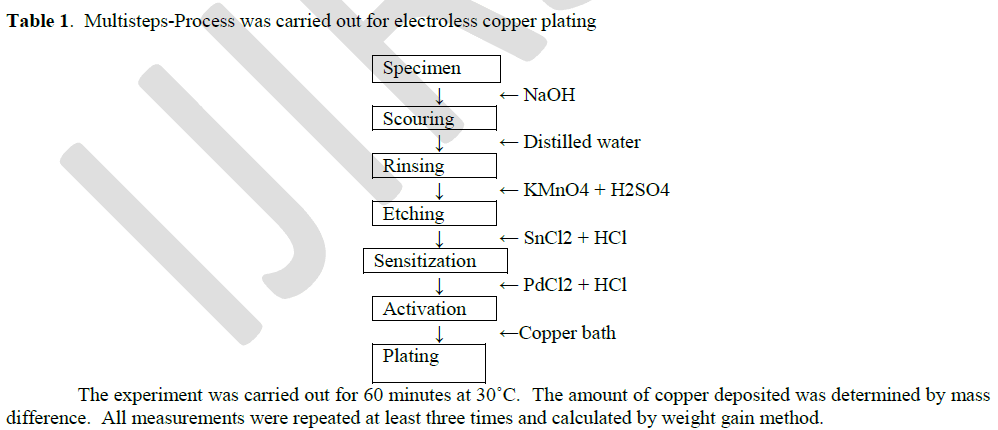 |
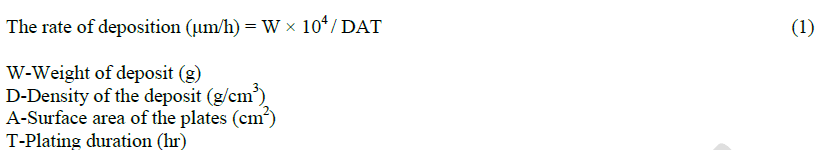 |
IV. RESULT AND DISCUSSION |
Physico-Chemical analysis |
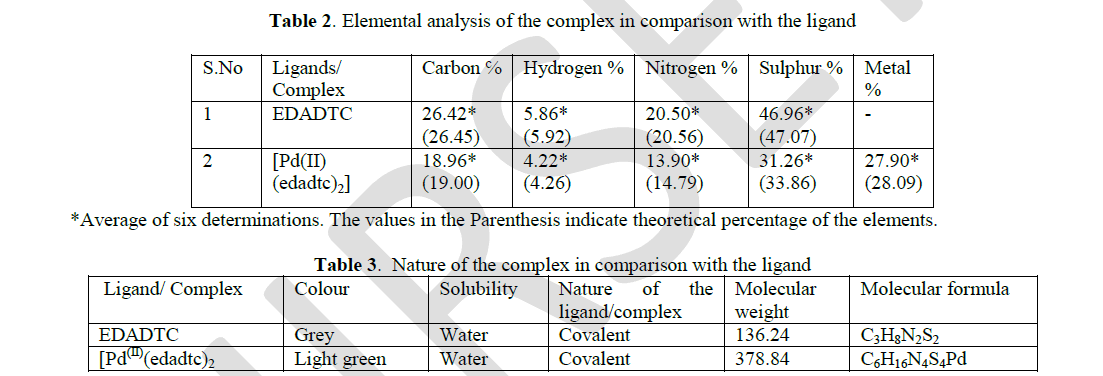
| The ethylenediamine complex of Pd(II) was found to be pale green and found to be soluble in water. The hydrophilic moieties in this complex are responsible for their water solubility. In water, the conductance of 10-3M solution of all the complex prepared in deionised water was found to be < 10 M ohm-1 cm-2 mole-1. This shows that the complex are covalent in nature. The elemental analysis of this complex shows that the percentage composition of carbon, hydrogen, nitrogen, oxygen and sulphur corroborates with the theoretical value. The following results prove that the EDADTC and complex of palladium are bi-chelate. |
 |
Electronic spectral analysis |
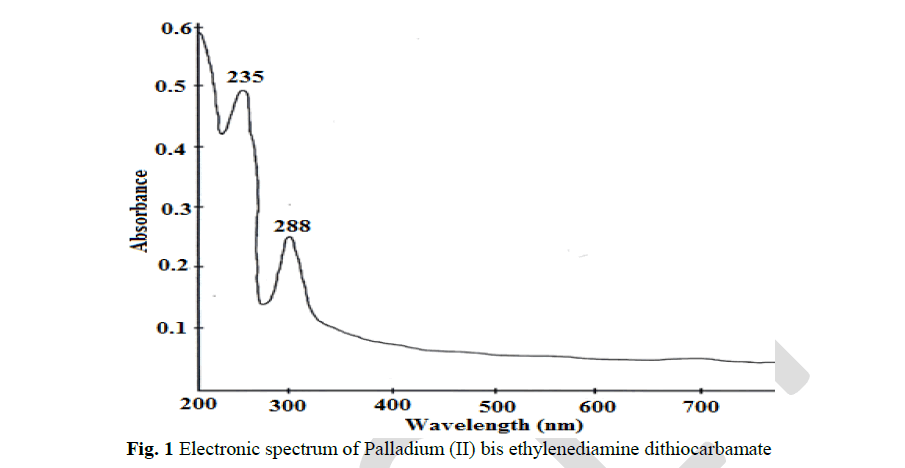
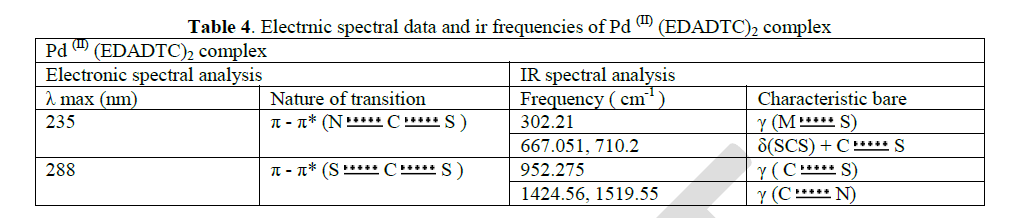
| The pale green coloured ethylenediamine dithiocarbamate complex of Pd(II) showed a shift in λ max values at 235 nm and 288 nm in comparison with the ligand. This shift is due to the effect of complex formation. These λ max values is the UV region are corresponding to π-π* transition of S C N and S C S chromophores respectively. |
 |
IR spectral analysis |
| The thioureide band between 1424.56 cm-1 and 1519.55cm-1exhibits a partial double bond character between carbon and nitrogen. A single strong band observed at 952.275 cm-1 is attributed to the stretching vibration of C S bond. The stretching vibration of C S +S(SCS) band are observed at 710.2 cm-1 and 667.051 cm-1 In addition to the characteristic band of the ligands, a strong band of metal - sulphur linkage is observed at 302 cm-1 which confirm the formation of complex. |
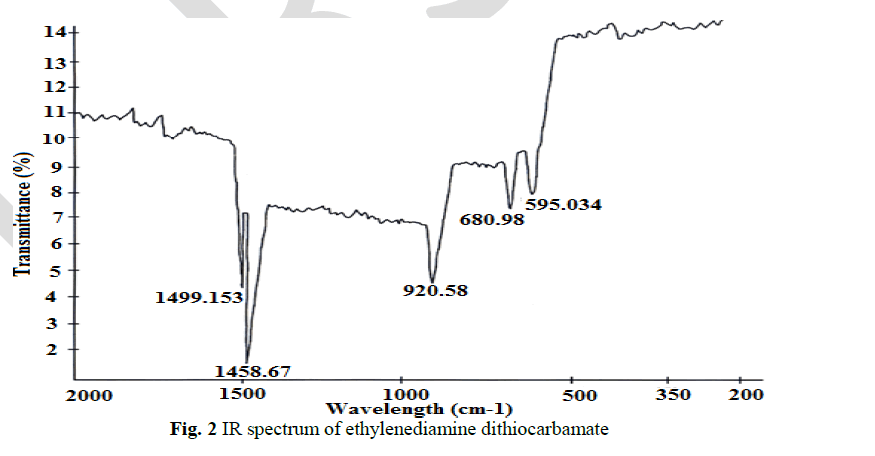 |
 |
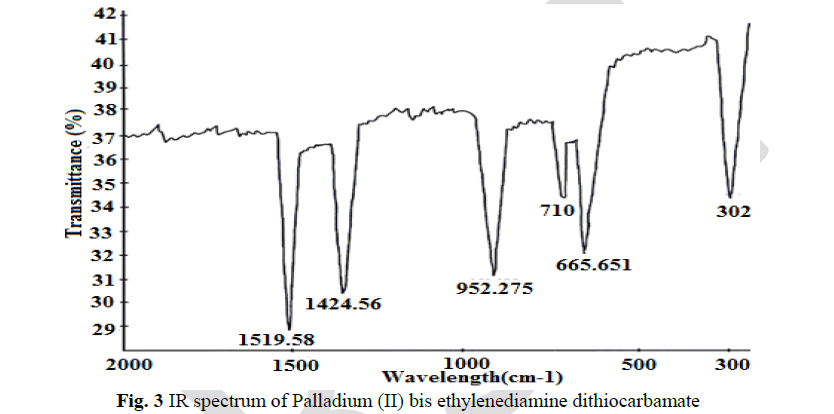 |
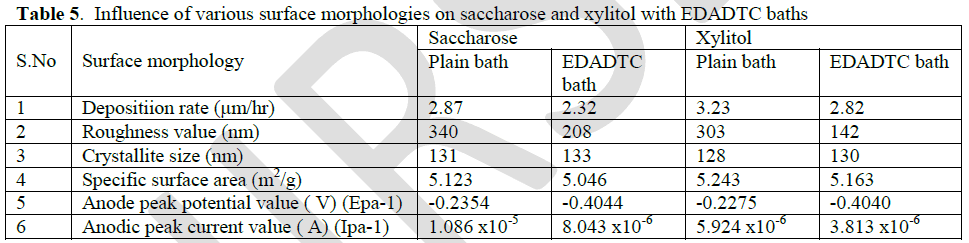
| The absence of stabilizer unstable at longer experiments and the plating process is poorly reproducible. [25] So EDADTC was used as stabilizers in electroless copper methane sulphonate bath. The copper deposits on EDADTC bath are sufficiently stable at 1ppm, reaches 2.32 μm/h on saccharose bath and 2.82 in xylitol bath, at very low concentration there was no significance difference in rate of deposition. |
| The effect of temperature of electroless copper deposits on saccharose- and xylitol containing methane sulphonate plain bath, and EDADTC bath are studied. In plain bath (ie) methane sulphonate bath without stabilizer the temperature increases deposition rate also increase. But the bath is stable up to 40Ãâ¹ÃÅ¡C, thereafter less stable at 45Ãâ¹ÃÅ¡C and unstable when reaches 50Ãâ¹ÃÅ¡C. The copper deposits on EDADTC bath is inhibit more than the plain bath and the bath are stable up to 45Ãâ¹ÃÅ¡C and unstable at higher temperature. There is a turning point at 45Ãâ¹ÃÅ¡C modify the stability of EDADTC bath. |
Surface morphology |
| Atomic force microscopy (AFM) studies explain the colour of EDADTC used copper deposits are dark brown with saccharose and semi bright with xylitol bath. The following AFM images, (a) the topography of copper deposits, (b) 3-D images and (c) surface area were explain the roughness of the deposits. |
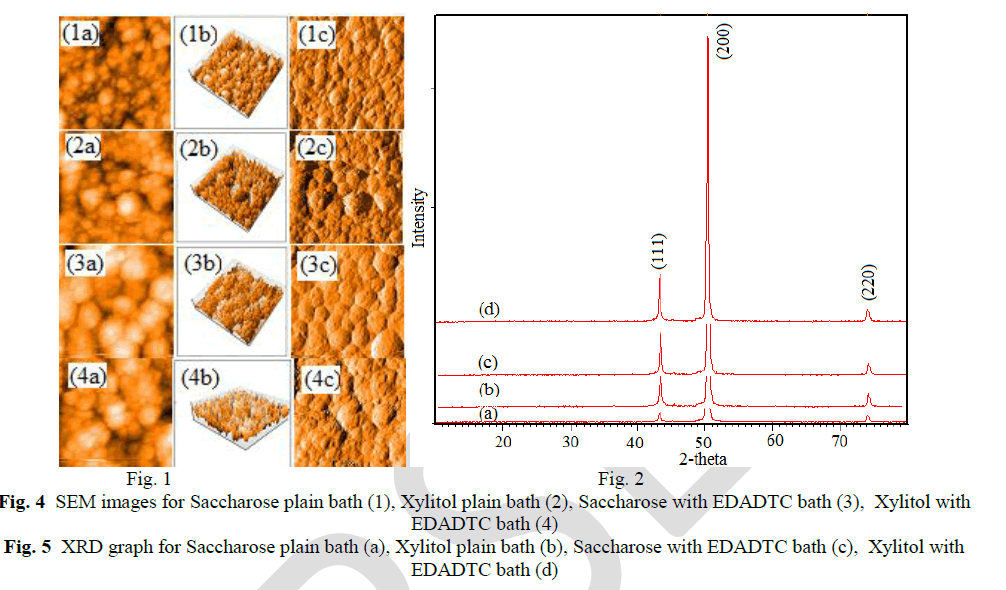 |
Structural property |
| The crystal orientations and lattice parameters were studied by XRD. The peaks of the pure copper metal indicate preferred orientation of (111) plane. But Lee et al [26] concluded that copper methane sulphonate bath furnished more number of copper ions, because of high conductivity and solubility leading to (200) plane. The copper deposits on saccharose and xylitol containing methane sulphonate plain baths with EDADTC bath are give the preferred orientation of (200) plane. The crystallite size of the copper deposits can be estimated by the use of Debye-Scherer’s equation. [27] |
 |
Electrochemical studies |
| Based on CV studies, the inhibiting properties of the stabilizer result in low anodic peak potential value. The low energy oxidation process is enhanced by the stabilizers. The appearances of the sharp peaks indicate that the rate of oxidation is high. The high anodic peak current value also indicates that the stabilizer inhibits the deposition of copper. |
| Table [5] indicates the inhibiting property of EDADTC on saccharose and xylitol baths. Because it shows low anodic peak potential value than respective plain baths. |
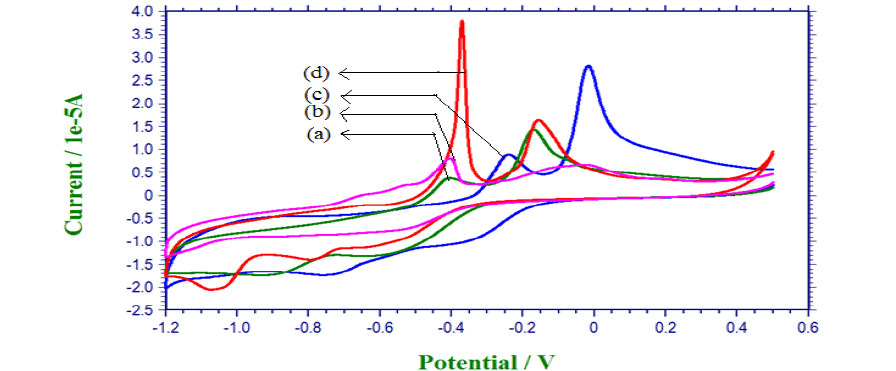 |
| Fig. 6 Voltammogramme for Saccharose plain bath (a), Xylitol plain bath (b), Saccharose with EDADTC bath (c), Xylitol with EDADTC bath (d) |
 |
V. CONCLUSIONS |
| The molecular formula of the [Pd(II) (edadtc)2 ] complex is C6H16N4S4Pd. It is pale green and the molecular weight of the complex has been found to be 378.84. The molecular weight determination and rapid elemental analysis supports the fact that the complex was water soluble and are bi-chelate. The electronic spectral analysis and IR Spectral analysis supports the fact that complex was covalent and very stable. |
| The environmental-friendly safe saccharose and xylitol containing copper methane sulphonate with EDADTC forming stable bath in alkaline solution. AFM and SEM studies have been carried out for EDADTC bath and confirmed the deposits was dark brown colour, increase the grain size. XRD studies also support the crystallite size and specific surface area of copper deposits. The copper deposits had a higher (200) plane orientation. The quality and quantity of the copper deposits were investigated by cyclic voltammetry studies. Moreover xylitol based deposits are produces more compact and fine in structure than saccharose bath. |
References |
|