ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
C. ZIDI1, A. JAMRAH2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The objective of this study is to develop a supported liquid membrane (SLM) system containing a mixture of solvating extractant (tributyl phosphate (TBP)) and suitable organic solvent (kerosene) for the treatment of liquid solutions involving phenol or vanillin. Previous studies of liquid-liquid extraction (LLE) have been conducted to determine the feasibility of phenol and vanillin extraction through a MLS process. The complexing properties of TBP were studied in LLE as well as in the transport through a SLM. On the other hand, the use of TBP- kerosene mixture as organic phase leads to a quantitative extraction of phenol and vanillin. Experimental study results of phenol facilitated transport through a SLM show that the best transport performance was accomplished with a source aqueous solution at pH 2 and receiving aqueous solution of 0.2 M NaOH knowing that the transport efficiency decreases with time during five days of continuous operation. The study of vanillin facilitated transport through a SLM containing TBP- kerosene mixture has also been described. The optimum extraction of vanillin was accomplished using 20 % TBP (v/v) in kerosene as organic membrane mixture, an aqueous source at pH 1 and a receiving aqueous solution of 0.5M NaOH. The extraction efficiency of vanillin was stable at around 63% with the developed SLM system for 5 days of continuous run.
Keywords |
| Supported Liquid Membrane (SLM), Liquid-Liquid Extraction (LLE), Tributyl phosphate (TBP), Phenol, Vanillin, stability. |
INTRODUCTION |
| Numerous techniques available for the treatment of effluents containing phenol can be divided into two main categories: destruction and recovery methods [1]. Among destruction methods are cited: biodegradation, incineration, ozonation in the presence of UV radiation, electrochemical oxidation [2] and moist air oxidation [3]. Whereas recuperation or recovery methods include liquid-liquid extraction (LLE) [4]-[6], adsorption and electro-adsorption on activated carbon [7], [8], ion exchange in resins [9], membrane processes such as pervaporation [10], supported liquid membranes [11] and emulsion membranes [12], [13]. On the other hand, several methods for determining vanillin and ethylvanillin from several food samples or vanilla extracts have been described involving the use of gas chromatography/mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC), capillary electrophoresis (CE) and amperometric detections [14]. In the work reported here, a new method for the determination of vanillin in aqueous samples is proposed and involves the use of a SLM. Unlike LLE, liquid membrane processes combine extraction and stripping in one step and do not obey the characteristics of the equilibrium mass transfer where the separation is not limited by equilibrium conditions. Among the liquid membrane processes, liquid membrane emulsion (MLE) and supported liquid membranes (SLM) have been extensively studied since 1970s. The SLM process comprises a microporous membrane support impregnated with an organic liquid (or also extractant dissolved or diluted in an organic solvent carrier) for separating a source of a receiving aqueous phase. The SLM process offers many advantages such as low investment and operating cost, low power consumption, small amount of liquid membrane and simple operation and large-scale use. This study refers to the LLE of phenol and its recovery through a SLM system containing TBP- kerosene mixture as organic liquid membrane, as well as the study of vanillin LLE by TBP- organic solvents mixtures and its facilitated transport through a SLM system using the TBP- kerosene as organic membrane phase. |
II. MATERIALS AND METHODS |
| A. Carrier Synthetic carriers must be poorly soluble in both aqueous extramembraneous phases but, easily soluble in the organic membrane phase. They also need to form a complex of moderate stability with the transported species, for easy decomplexing in the receiving side. The tributyl phospahte (TBP) was used in this study, as solvating extractant for the extraction of phenol and vanillin from an aqueous phase. TBP used throughout this studiy is a product of Fluka (purity greater than 97 %). B. Solvents For this system, hexane (Fisher Scientific product, purity greater than 99%), decane (provided by Merck), the octan-2- ol (Fluka product, purity is about 98%) and kerosene (Fluka product) were used as solvents because of their characteristics ensuring good transport efficiency and stability of the SLM. The solvents used must have a high boiling point. In addition, these solvents must be extremely hydrophobic to avoid their dissolution in the extramembraneous aqueous solutions [15]. A 2 ml of an aqueous solution incorporating 200 mg L-1 of phenol (Fluka, Switzerland) adjusted at a pH of 2 with few drops of H2SO4, 1 mL of TBP and 1 mL of studied organic solvent were mixed in glass tubes capped and stirred at 30 rpm for 24 h (suggested to be sufficient to reach equilibrium time) at 25 ± 1 ° C. C. Experimental procedure of liquid-liquid extraction A 2 ml of an aqueous solution incorporating 50 mg L-1 of vanillin (99% pure, Sigma -Aldrich, USA) adjusted at a pH of 1 with also few drops of H2SO4, 1 mL of TBP and 1 mL of studied organic solvent were mixed in glass tubes capped and stirred at 50 rpm for 24 h (suggested to be sufficient for equilibration time) at 25 ± 1 ° C. The mixture was then centrifuged and the two phases were well separated. The analyte concentration was determined by UV-visible spectrometric technique (Perkin -Elmer Lambda 20 double- beam spectrophotometer deviation). The wavelengths of maximum absorption determined from phenol and vanillin calibration curves are ïÃÂì= 270 nm and ïÃÂì= 232 nm, respectively, for a concentration range from 0 to 60 mg L-1 (linearity area). The percentage of extraction % E [15] was calculated from the concentration of the element to retrieve before ([XOH ] aq , i) and after ( [XOH ]aq) extraction: |
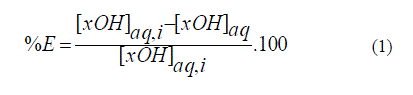 |
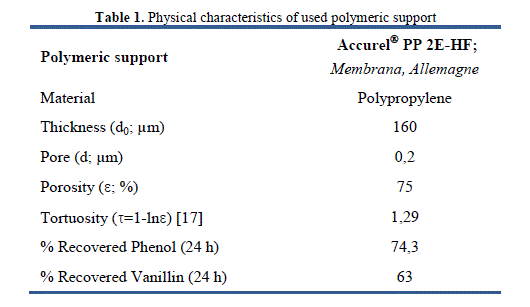
| consisting of two Teflon reservoirs (100 mL each); respectively [16], both source and receiving reservoirs were agitated by a rotating magnetic bar (600 rpm at 25 ± 1 °C) to avoid concentration polarization conditions at membrane interfaces and in solutions. The two compartments were covered with waxed paper Parafilm® to minimize evaporation phenomenon. Transport was always conducted at a room temperature. The microporous polymeric support used in this system is polypropylene film (PP) type Accurel® PP 2E-HF (provided by Membrane (Germany)). Physical parameters (thickness, porosity and average pore diameter) of the latter are listed in Table 1. |
 |
| Source phase: to study the transport of phenol and a derivative thereof namely vanillin through a SLM, source phase was buffered to a pH sufficiently acidic. This solution was obtained by dissolving the analyte in the ultra pure water (MilliQ more column, Millipore) at a defined concentration (200 mg L-1 for phenol and 50 mg.L-1 vanillin), and some drops of sulfuric acid H2SO4 to acidify the solutions. Receiving phase: the stripping solution must be basic in order to dissociate the complex formed between analyte (phenol or vanillin), carrier (TBP) and solvent (kerosene). Indeed, the analyte-carrier-solvent complex formed at source phase-membrane interface is dissociated at the other membrane-receiving phase interface to form sodium phenate or sodium vanillate due to the contact with 0.2 mol L-1 NaOH solution (Carlo Erba produt). Impregnation techniques: the polymeric support is impregnated by adsorption. Indeed, a few drops of organic membrane solution were poured directly on the microporous polymeric support. Then, the solution diffused into the pores of the material (24 h was a sufficient time). Penetration of the organic liquid was thus carried out very easily because the surface tension of the impregnation liquid was less than the critical tension of the polymeric support which was about 30N.m-1. Sampling techniques: the experimental cell used was made of Teflon PTFE, 1 mL of each of the two aqueous phases were removed without being replaced. Therefore, transport properties of the system were not influenced. Collected samples were stored in glass vials and then diluted with accuracy to allow the determination of phenol and vanillin by UV -Visible. All experiments were carried out in duplicates and variations in measurements were almost about ± 5%. |
III. RESULTS AND DISCUSSION |
| A. Liquid liquid extraction of phenol and vanillin 1) Extraction efficiency Four different solvents namely, decane, hexane, octan-2-ol and kerosene were mixed with TBP (50%v/v). The extraction efficiency E% and the distribution coefficient D, expressed as log D, are summarized in Table 2 and Table 3; respectively. It is appropriate to note that all the tested solvents ensure quantitative extraction of phenol more than 88% and vanillin greater than 98%. The best extraction efficiency was obtained for TBP- octan-2-ol mixture selected as the organic phase in the case of phenol. Similarly, Kujawski et al. [17] showed that the methyl-tert-butyl ether (MTBE) has been suggested as the best extraction solvent of phenol, based on solvent extraction membrane system, compared to the mixture of hydrocarbons and cumene mixture. Besides, Jiang et al. [18] have determined that alcohols are more effective for the removal of phenol than amines and carboxylic acids. In addition, these authors reported tçhat alcohols having longer carbon chains (C8, C11 and C12) have better extraction efficiency than those with shorter chains (C5, C6 and C7). |
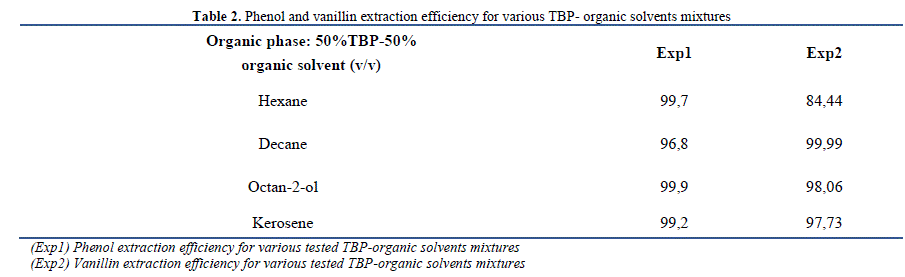 |
| By comparing the distribution coefficients of phenol listed in Table 3 with those reported in the literature using some common solvents without the addition of TBP, it can be concluded that this addition improves the extraction efficiency (synergistic effect). In fact, a rise from a logD of 1.44 using pure octan-2-ol [19] to a logD of 1.76 after dissolution of TOPO in octan-2-ol and to a logD of 3.00 in the presence of TBP-octan-2-ol mixture as organic phase was detected. Besides, Cichy and Szymanowski [20] showed that the extraction of phenol by only aliphatic kerosene is low (D = 0.12) contrarily to TBP-kerosene mixture (D = 124.00) tested in this study. |
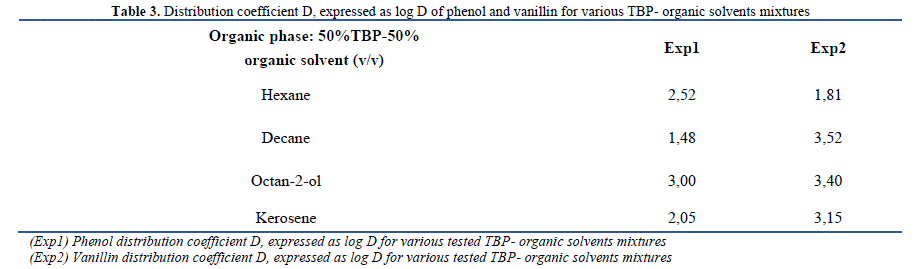 |
| In the case of vanillin, the best extraction efficiency occurs with TBP-decane as organic phase mixture, followed by octan-2-ol, kerosene and hexane. Hexane shows the lowest extraction efficiency compared to tested solvents, this result is confirmed by Tarabanko et al. [21] which declared that the extraction of vanillin by octylamine-based systems decreases with the use of additional anti-solvents, eg, hexane, toluene, benzene, dichloroethane and chloroform. The distribution coefficient of vanillin , expressed as log D , improved from -0.7 in presence of pure hexane [22] to 1.81 using 50% TBP- 50% hexane mixture (v/v) as organic phase . Similarly, logD varies from 1.19 as determined by Noubigh et al. [23] using octan-1-ol in slow stirring method to 3.4 using TBP-octan-2-ol mixture. The addition of TBP improves vanillin extraction efficiency which can be considered as suitable complexing agent for the recovery of vanillin from aqueous solutions [20]. Vanillin distribution coefficients obtained are higher than those found in literature using other systems [22]-[25]. Indeed, Tarabanko et al. [22] demonstrated that vanillin distribution coefficients increase with the concentration of octylamine to values not exceeding 2.78, in a pH range varying from 8 and 10. Fries et al. [22] studied the extraction of vanillin dissolved in water with toluene as organic phase using suitable rectangular microchannels made of polydimethylsiloxane (PDMS), a distribution coefficient of 0.62 was been reached. Moreover, Claudio et al. [24] determined vanillin distribution coefficients using improved -liquid-based aqueous two-phase systems (ATPS). Three main parameters were evaluated in the process of vanillin distribution: anionic and cationic structure of the Ionic Liquid (IL), equilibrium temperature and available vanillin concentration in the overall system. Under all systems and tested conditions, vanillin preferentially migrates to the IL rich phase with distribution coefficients ranging from 2.7 to 98.7 whereas these factors are beyond 1000 in our study case for all tested mixtures (TBP-organic solvents) with the exception of hexane. 2) Kinetics The extraction kinetics of phenol and vanillin using TPB as dissolved extractant in various organic solvents has been studied following the experimental procedure detailed earlier. Aqueous and organic phases were mixed with different contact times (0-24 h). Variation of the extraction efficiency as a function of time is shown in Fig. 1.1. and Fig. 1.2. |
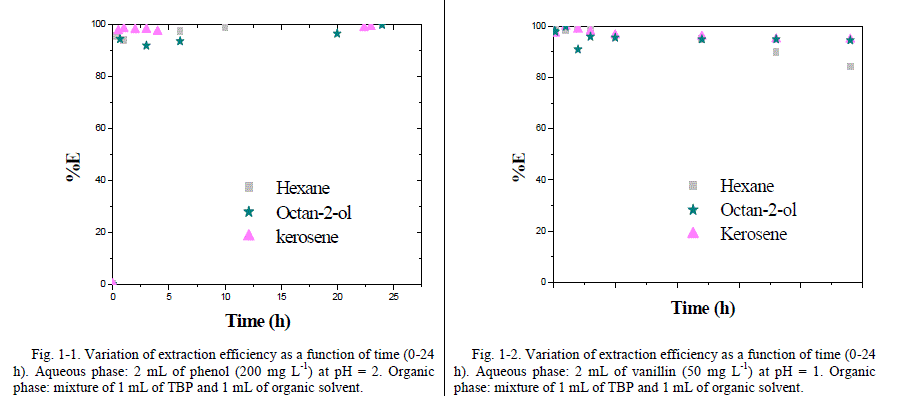 |
| From these analytes variation plots, it was observed that the extraction percentage is more or less constant after few minutes of contact time. In various cases and after only 5 minutes of phase equilibrium, the removal of phenol reached over 82 % and vanillin extraction is about 96%. Contact time extension beyond 5 minutes has only a slight effect on analytes extraction percentage. Results ensure that 5 minutes as suggested time is considered to be sufficient to achieve apparent equilibrium. Kerosene was used to study the facilitated transport of phenol and vanillin through a Flat Sheet Supported Liquid Membrane (FSSLM) system involving TBP as extractant [26]. Knowing that, kerosene is commonly and widely used for the development of SLM systems. B. Facilitated transport of phenol and vanillin 1) Kinetics Changes in concentrations of species to extract in both source and stripping compartments are illustrated in Fig. 2-1 and Fig. 2-2. It should be noted that phenol or vanillin concentrations in source decreases for the first few hours of the transport to reach a plateau, while concentrations of analytes in the receiving phase increase linearly to reach a plateau [27]. More than 67% of species initially present in the feed reservoir was transferred to the receiving reservoir after 24 h of transport through a SLM. |
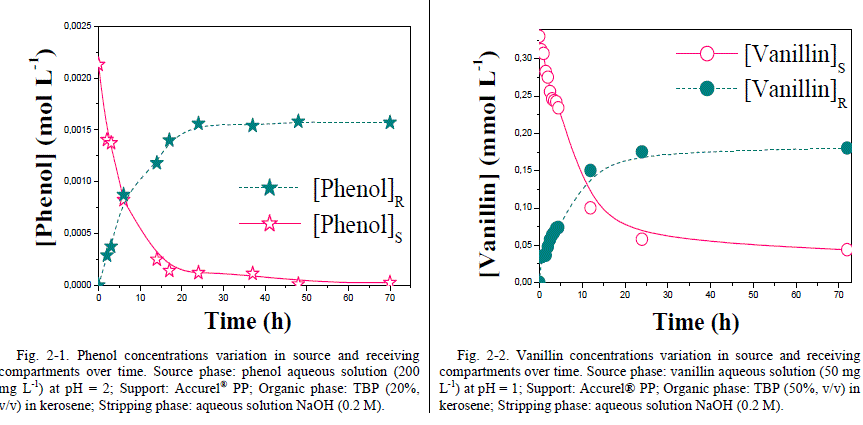 |
| Moreover, the increase in membrane surface area could increase the efficiency of the transport system. It can also be observed that there were more species extracted from the source than re-extracted in the receiving phase. This can be explained by the fact that the experiments were performed under optimal conditions (NaOH and TBP optimal concentrations) causing the accumulation of certain amount of complexed species (xOH.TBP)org in the organic membrane phase. As shown in the above figures, a typical image of facilitated transport from source phase to receiving phase of our selected species is observed. The transport driving force is the concentration gradient of the complexed species formed in the membrane phase between the analyte and the carrier dissolved in kerosene (xOH.TBP) org. Indeed, complexed species formed in source-membrane interface, diffuse through the membrane to be dissociated at membrane-receiving phase interface via formation of sodium phenate or sodium vanillate which are insoluble only in organic phase. Accordingly, Sodium phenate or sodium vanillate do not retro-diffuse in the membrane and the transport driving force is maintained (complexed species concentration gradient) [28], [29]. 2) Membrane stability To study the membrane long-term stability, transport efficiency of phenol for 5 days of continuous run under optimal conditions and without re-impregnation of the membrane, was examined. The percentage of phenol transported was measured every 24h. Similarly, the transport efficiency of vanillin for a period of five days of continuous operation under optimal conditions without re-treatment of the membrane was also examined. Depleted source and enriched receiving phases were replaced with fresh solutions after every 24 hours of continuous run (Fig. 3). Indeed, after three days of continuous experimentation, a 52% decline of phenol transport efficiency was observed using Accurel® as polymeric support. This decline in transport efficiency may be due to the loss of organic liquid membrane support pores [30]- [32]. Jaber et al. [33] studied the transport efficiency of phenol over two weeks time period through SLM containing functionalized polyorganosiloxane. Transported phenol concentration at equilibrium is about 100, 80 and 70 % of phenol initial concentration for a freshly prepared membrane and one week storage intervals, respectively. |
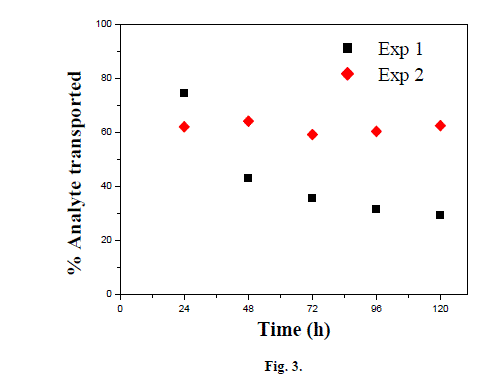 |
| The percentage of vanillin transported was measured for 24 hours, then impoverished source and enriched receiving vanillin solutions were replaced with freshly prepared ones. From Figure 3, the SLM system used does not have a dependent negative trend of the time. It can be concluded that after five days the membrane retains its original performance. This allows higher expectations for the developed SLM life which is commonly considered a critical weakness of SLM systems [34], [35]. Luque et al. [36] used the same supported di-n-hexyl ether membrane for more than a month of daily work to determine vanillin in food samples with amperometric detection, but between successive determinations the membrane was regenerated with 4 drops of organic solvent. Besides, Avila et al. [14] have developed a piezoelectric sensor having a flow supported liquid membrane system modified with a molecularly imprinted polymer for the determination of vanillin in food samples. The membrane was regenerated between analyses by adding 0.5 mL of organic solution to the membrane for 5 minutes. |
IV. CONCLUSION |
| Studies of Liquid- Liquid Extraction (LLE) were conducted to determine the feasibility of extracting phenol or vanillin by SLM system. The complexing properties of TBP were studied in LLE and transport through a SLM. The results of LLE shows that time does not exceed few minutes considered to be necessary and sufficient to achieve equilibrium in all bi-phasic studied systems. On the other hand, the use of TBP-kerosene mixture as organic phase leads to a quantitative extraction of phenol as well as vanillin from water. Therefore, this mixture was elected as the best organic liquid membrane for phenol and vanillin recovery (facilitated transport) through a SLM system. Various operating parameters that affect transport efficiency through SLM have been detailed. The study of phenol facilitated transport through a SLM using TBP-kerosene mixture as organic liquid membrane was examined. Experimental results show that the best performance of transport was accomplished with an aqueous source at a pH of 2 and 0.2 M NaOH aqueous receiving solution. It was also observed that the efficiency of transport through the developed SLM decreases with time during five days of continuous run. In parallel, the study of vanillin facilitated transport through a SLM containing the same mixture TBP-kerosene as organic liquid membrane was described. The optimum extraction of vanillin was accomplished using 20%TBP (v/v) in kerosene as organic membrane mixture, an aqueous source at a pH of 1 and a receiving aqueous solution 0.5M NaOH. An extraction efficiency of vanillin stable around 63 % with the SLM developed system was obtained for 5 days of continuous testing. All this work has provided possible industrial application of SLM systems described, especially when the effective membrane area exposed can be significantly increased by using a SLM system with hollow fibres configuration. This system can be very useful for the recovery of phenol or vanillin from industrial effluents even at high concentrations. However, the use of such system could apply an effluent pre-treatment depending on its initial characteristics. |
References |
|