ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Aveen H. Matti1, Kafia M. Surchi2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Batch experiments have been performed on samples of synthetic homoionic sodium form NaY zeolites to measure their cation exchange capacity (CEC) and the kinetic parameters associated the exchange process. The kinetic data of the ion exchange of homoionic sodium form NaY zeolite/ Ca aqueous solutions systems at different temperatures was best fitted to Pseudo second order kinetic model. The results indicated that diffusion is the only mechanism that describes mass transfer from the particle surface to the exchangeable site, and intraparticle transport is the only rate –limiting step in the process of cation exchange.
Keywords |
| Cation exchange capacity, Cation Exchange kinetic models, mass transfer mechanism, NaY zeolite. |
INTRODUCTION |
| Zeolites are crystalline microporous aluminosilicates, built up of a 3-dimentional framework of [SiO4]4- and [AlO4]5- tetrahedra, linked by sharing oxygen atoms, and weakly bonded (readily exchangeable) cations and water molecules in the pores and voids of the structure. Industrialization is the back bone of every nation’s economy and the enhancement of citizenry well-being [1]. Zeolites are commonly used in environmental remediation for selective removal of toxic materials by cation exchange process. The CEC results from the presence of loosely bound cations of alkali and alkaline earths elements, often called exchangeable cations, in the structure of the zeolites. These loosely bound cations are easily exchanged when zeolites are in contact with solutions of saturating or indexing ions. The cation exchange rate can be limited by the speed of ion motion towards the zeolite. Zeolites are used in environmental remediation due to their high ion exchange capacity, molecular-sieve properties, which can be easily modified to make them selective of certain ion sizes [2, 3]. |
| CEC is directly related to the amount of aluminum present in the framework. Properties such as Si/Al ratio, specific to the cationic species in solution and the anionic associated to them, solvent, pH, and temperature must be observed for good exchange efficiency. The exchange process is described as [4]: |
| M1 (Z) + M2(S) ↔M 2 (Z) + M1(S) (1) |
| WhereM1 (Z) is the exchangeable cation present in zeolites Z, and M2 is the saturating ion in solution. Many methods are known for the determination of CEC. Ammonium acetate, BaCl2 or CaCl2 compulsive exchange, tetra ethylene amine, complex of Cu(II) with triethylenetetramine, Co hexamine trichloride and methylene blue. CaCl2 and ammonium acetate method was the most widely used methods. |
| Ion-exchange kinetics, though of considerable importance in zeolite applications such as catalysis and in detergent action, has not been as extensively studied because of the complexity of the process. Diffusion of ions can be rate limiting within the crystal (particle-controlled diffusion) or in passing through the zeolite-fluid boundary (surface diffusion), with the latter becoming more important for smaller crystallite size. Within the crystal, diffusion is promoted by concentration gradients as well as influenced by electrical potential gradients due to the charge density differences of the exchanging ions [5-6]. The methods of ammonium acetate saturation (AMAS) and methylene blue absorption (MBA) are commonly used to measure the CEC of finely crystalline materials. |
RELATED WORK |
| A comprehensive study of alkaline earth ion exchange in zeolites X and Y over the temperature range of 5–50oC was made by the ion exchange capacities of Na for Cr/K, Cr/Mg and Cr/Ca in Y and X zeolites by using breakthrough curves [5]. |
| Liu et al [7] studies NaY zeolites which are in-situ synthesized from coal-based kaolin via the hydrothermal method. The ion exchange isotherms of Cr3+, Mg2+, Ca2+ and K+ in binary mixtures (Cr/K, Cr/Ca, Cr/Mg) using NaY zeolite at 30, 45 and 60oC is reported by Barros at 2005 [8]. They found that ion exchange behavior of all systems seems to be very dependent on temperature, nature and interaction of in-going cations. This investigation concentrates on examination of kinetics of CEC of different zeolite samples using empirical kinetic models, comparison of their CEC and establishing the exchange mechanism of ions included in the ion exchange process. |
MATERIALS AND METHODS |
| Zeolite Synthesis |
| In our previous investigation a simple and easy method was employed to synthesize of homoionic sodium from of Nay Zeolite to measure its CEC kinetically [9,10]. |
| Kinetics of CEC measurement |
| Experiments have been performed on sample of synthetic homoionic sodium from of Nay zeolite prepared to measure its CEC and the kinetic parameters associated the exchange process [11]. |
| Batch model was selected because of its simplicity and reliability. The ion exchange experiments were carried out in a series polyethylene tubes containing zeolite and CaCl2 suspensions at constant temperatures. |
| Preliminary studies of CEC for NaY zeolite indicated that the process was characterized by a rapid change during 12- 14 hs of contact time followed by more than one week to be sufficient to achieve the equilibrium. |
| The CEC measurement experiments were conducted by equilibrating 5 g of the prepared zeolite, in 50 ml centrifuge tubes, with 25 ml of 2M solution of CaCl2. The mixtures were shaken continuously for 9 days at temperature controlled (303, 318 and 328 K) water bath with shaker at 185 rpm. When equilibrium time was achieved the supernatants were removed by centrifugation at 3500 rpm for 20 minutes. The supernatants of four replicates were collected together and analyzed spectrophotometrically for Na+1 and Ca+2. |
CALCULATION |
| Based on the results for concentrations obtained from the examination of ion exchange kinetics, the amount of Ca2+ bound at selected solid/liquid contact times qt and at equilibrium, qe, has been calculated from [12]: |
| qt= (C0-Ct)V/m (2) |
| The exchange capacity (qe) was determined using the mass balance expression: |
| qe= (C0-Ce)V/m (3) |
| Where Co and Ct are the Ca2+ concentrations in liquid phase at the initial and time t (in ppm) respectively, m is the weight of the zeolite (in g), and V is the volume of the solution (in ml). |
RESULTS AND DISCUSSION |
| Cation exchange Process |
| Kinetics Exchangeable cations are those that can be changed by a cation of added solution, or that is, any added cation will exchange the zeolite cation. The basic exchangeable cation in NaY zeolite is Na1+, which was exchanged by Ca2+.The ion exchange kinetics of Na Y zeolite/Ca aqueous solution system was done for the studied NaY zeolites. |
| Effect of contact time |
| Examination of ion exchange kinetics of Ca2+ was performed by means of the batch method in Na Y zeolite/ Ca aqueous solutions systems. Contact time is inevitably a fundamental parameter in all transfer phenomena such as cation exchange process. |
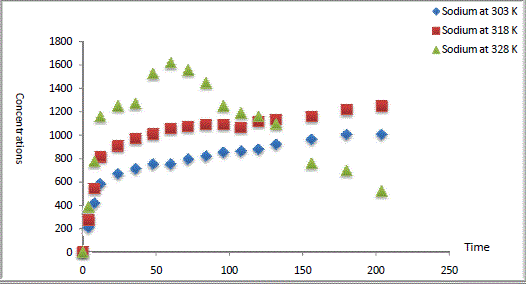
| The experimental results of CEC kinetics were illustrated in Fig. 1 represents the data obtained from Na1+ exchanged by Ca2+ in Na Y zeolite. The data showed that a contact time of 9 days was required to achieve the equilibrium. The ion exchange of Ca2+ shown is rapid at initial times with prolonged slower exchange until equilibrium. During the first hours of Na Y zeolite/Ca aqueous solutions contact (12-24 h), the rate of cation exchange is found to be very rapid, and thereafter, the rate of metal ions exchange decreased. |
| During the initial stage of the process, a large number of Na1+ is available for exchange. After lapse of sometime, the ingoing metal ions (Ca2+) are binded into the mesopores that get almost saturated with in-going metal ions during the initial stage of exchange. Our results were in good agreement with that reported in literature [13-14]. |
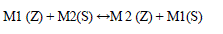 |
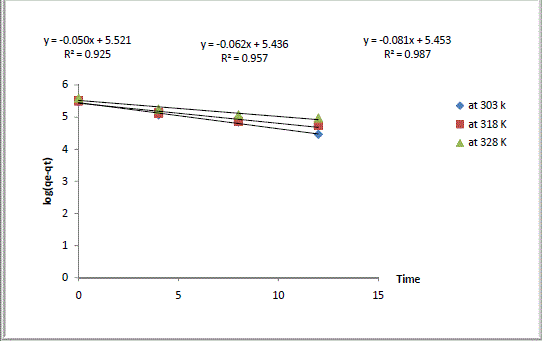
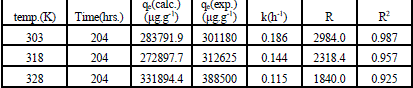
| Where qe and qt were defined previously, k1 is the pseudo first-order rate constant of the process (h-1). The values of k1 and qe were calculated from the slope and the intercept of the lagergren plot of log (qe - qt) versus t as shown in Fig. 2. The pseudo first-order rate constant k1, amount of Ca2+ exchanged calculated at equilibrium ,qe, the experimental values of qe, exchange rate, R, and the correlation coefficients ,R2, obtained from the pseudo first-order rate model were all gathered in Table 1. The large differences between the experimental and the calculated values of qe indicated that pseudo first-order mechanism is not fully followed in this process. |
 |
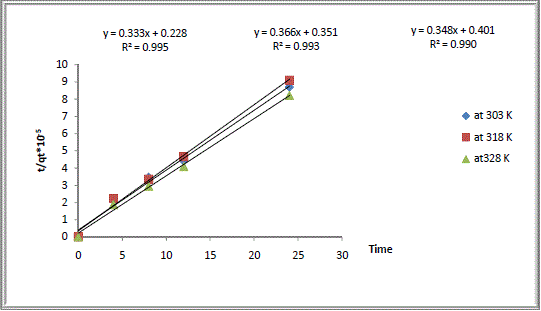
| Values of both constants k2 and h together with values of qe can be calculated from the intercept and slope of the line obtained by plotting t/qt versus t. The values of model parameters (k2, h, and qe), calculated values of qe as well as experimentally measured values, and the pseudo second order regression of coefficients of determination (R2) were all given in Table2. |
| It can be seen that cation exchange kinetic of Na Y zeolite/ Ca aqueous solutions systems follows this model with correlation coefficients higher than 0.99 and the calculated values qe agreed well with the experimentally measured values. So the experimental kinetic data was best fitted to the pseudo second order kinetic model. Similar results have been reported in literature [16]. |
 |
 |
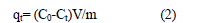 |
| Where α is an ion exchange initial rate (g.g-1h-1) and β is the revers rate (g.g-1h-1). The experimental results for cation exchange kinetic of Na Y zeolite/ Ca aqueous solutions system were analysed using the Elovich model, a plot of qt versus ln t, as shown in Fig.4. Values of qt, α, β, and R2 were all summarized in Table 3. |
| The values of correlation coefficient (R2) were ≥ 0969 which means that experimental results for cation exchange kinetic of Na Y zeolite/ Ca aqueous solutions systems were obey the Elovich model. |
| The general explanations for this form of kinetic equation involves a variation of the energetic of the exchange process with the active sites are heterogeneous in NaY zeolite/ Ca aqueous solutions system. It will be seen that applicability of the simple Elovich equation for the present kinetic data indicates that the Elovich equation was able to describe the kinetics of the ion exchange of Na Y zeolite/ Ca aqueous solutions systems. |
 |
 |
| Intra Particle diffusion kinetic model |
| When the Ca2+ solution is mixed with zeolite, transport of Ca2+ from the solution through the interface between the solution and the zeolite occurs into pores in the particles. There are four main stages in the ion exchange process [12]: (i) Ca2+ transfer from the bulk solution to the boundary film that surrounds the zeolite’s surface, (ii) Ca2+ transport from the boundary film to the zeolite’s surface, (iii) Ca2+ transfer the zeolites surface to active intraparticular sites, and (iv) exchange between the in-going Ca2+ and the available out-going Na1+ on these sites. |
| One or more of these four steps control the rate at which Ca2+ exchanged. Intraparticular diffusion of Ca2+ was characterized using the relationship between amount Ca2+ exchanged and the square root of contact time (t1/2). This relation is expressed as follows [18,19]. |
| qt = Kd t1/2 +C (8) |
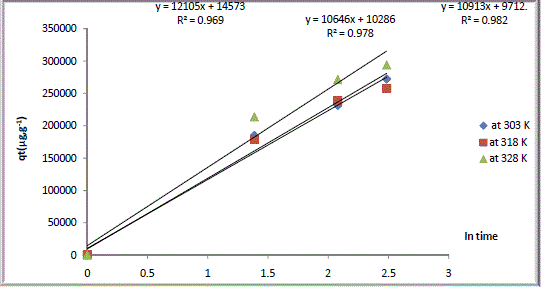
| Where Kd is the initial rate of intraparticlar diffusion (μgml-1 min-1/2) and C is the y- intercept. Fig.5 shows the diffusion of Ca2+ within the zeolite as a function of time. Values of intraparticular diffusion constants ( Kd) for the studied zeolite and at all temperatures are given in (Table 4 ). |
| Values of Kd showed that Ca2+ diffused quickly among the zeolite particles. Figure 4 shows that all lines passed through the origin (i.e. no y-intercept) which indicates that intraparticle transport is the only rate – limiting step in the process of cation exchange of Na Y zeolite/ Ca aqueous solutions systems. That indicates that diffusion is only mechanism that describes mass transfer from the particle surface to the exchangeable site. |
 |
 |
| Cation Exchange Mechanism |
| The rate of the ion exchange of Ca2+Na Y zeolite/ Ca aqueous solutions systems at different temperatures was rapid initially, but it gradually becomes slower with passage of time reaching a maximum in contact time of 9 days. The initial faster rate may be due to the availability of uncovered surface area of zeolite initially, since ion exchange kinetics depends on the surface area of the zeolite. It is likely that several mechanisms are responsible for the slow exchange reaction these may include diffusion, precipitation, and\or adsorption reactions on sites that have higher activation energy than the fast exchange sites. Moreover IntraParticle diffusion kinetic model parameters revealed that intraparticle transport is the rate – determining step in the process of cation exchange of Na Y zeolite/ Ca aqueous solutions systems [20]. |
| The ion exchange kinetics of Na Y zeolite/Ca aqueous solution system prepared by different procedures may vary widely in purity, chemical composition, crystal size, porosity, pores diameter and other properties, as well as their ion exchange capacity. Experiments have been performed on samples of synthetic zeolites prepared by different procedures to measure their CEC and the kinetic parameters associated the process. |
CONCLUSION |
| The CEC of Synthesized NaY zeolite was found to be dependent on contact time. The data was found to fit well to all with exceptional to the pseudo first order kinetic (Lagergren ) model. The kinetic of the ion exchange of Na Y zeolite/ Ca aqueous solutions systems at different temperatures was best fitted to Pseudo second order kinetic. The diffusion is only mechanism that describes mass transfer from the particle surface to the exchangeable sites of the studied samples. |
References |
|