ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| S. Ignatius Arockiam1, A. Peter Pascal Regis2 Department of Chemistry, St. Joseph’s College, Tiruchirappalli, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A solution route method was carried out for the synthesize of μ-peroxo-bis [aminebis (ethylenediamine) cobalt(III) ]perchlorate dihydrate. FT-IR and Electronic spectroscopic methods were used to characterize the synthesized complex. Biomimic oxidation of D-ribose by μ-peroxo complex has been studied kinetically in aqueous medium. Pseudo first order conditions has been followed to determine the rate of the reaction. The Oxidation reaction shows first order kinetics with respect to peroxo complex concentration and hydrogen ion concentration. The reaction rate points to negligible effect on increasing ionic strength. Potentiometric method was used to carry out the biomimic oxidation of the complex μ-peroxo complex. Thermodynamic and Arrhenius parameters were calculated and a plausible mechanism has been proposed.
Keywords |
| Kinetics, Oxidation, peroxo complex, D-ribose, Activation parameters |
INTRODUCTION |
| Living Systems are shaped by an enormous variety of biochemical reactions, nearly all of which are mediated by a series of remarkable biological catalyst known as enzymes [1]. The active catalyst contains the metal in its higher oxidation state so the transition metal ion with polydentate ligands as used as a model systems [2-3]. The oxidation of carbohydrates was widely studied by many researchers with different oxidants [4-9]. The peroxo complexes of cobalt (III) with different polyamines have been studied for effective model system. The present investigation on the oxidation of D-ribose with μ- peroxo complex has been studied extensively. |
EXPERIMENTAL METHODS |
| The reagents employed were D-ribose (Loba AR), H2SO4 and Na2SO4 etc were of AR grade. All solutions were prepared in doubly distilled water. The [(en)2(NH3)CoO2Co(NH3)(en)2](ClO4)4.2H2O was prepared by bubbling oxygen through a solution containing cobaltous nitrate, sodium perchlorate and the appropriate ligand mixture. The complex was characterized by FT-IR and electronic spectroscopic studies. Typical kinetic runs were carried out under pseudo first order conditions [D-ribose]>> [μ-peroxo complex].Appropriate amount of sulphuric acids, sodium sulphate, D-ribose and water was pipetted out in a double walled beaker provided with an inlet and outlet for water circulation from the thermostat set at desired temperature. |
| Requisite amount of μ-peroxo complex solutions was thermostated for half an hour and the kinetic reaction was started. The total volume of the reaction was 40 ml for the entire experiments. The cell [SCE/substrate-complex/pt] was used for the kinetics reaction with the reaction mixture and inserting the platinum and reference electrodes. The reaction mixture was stirred continuously using a magnetic stirrer throughout the experiment the emf of the cell was measured periodically using Equip-tronics potentiometer. |
| In order to study the effect of atmospheric oxygen, some experiments were carried out in an inert atmosphere by bubbling in nitrogen gas in the reaction mixture and it was ascertained that the velocity constants were reproducible within +2%. The rate of the reaction was not much variation between air and inert atmosphere; hence the entire experiment was done in air atmosphere. Sodium sulphate was used to keep the ionic strength constant in the reaction mixture. |
RESULTS AND DISCUSSIONS |
| Electronic spectrum |
| The single bridged μ-peroxo-bis[aminebis(ethylenediamine)cobalt(III)]perchlorate dihydrate shows an absorption band at 305 nm. The electronic spectrum shows that there is no characteristics transition in visible region, but the spectrum shows an intense change transfer band near 205 nm. This band is due to the transfer of electron from the peroxide to metal. This band clearly prove that the presence of a single bridge peroxo ligand in the μ-peroxo complex. |
| FT-IR spectrum |
| The Perkin Elmer RSI spectrometer was used for the FT-IR characterization of the μ-peroxo complex using KBr pellet in the wave length region 400-4000 cm-1 . The peaks at 3446, 3208, 2987 and 2385 cm-1 shows the presence of N-H stretching in the μ-peroxo complex. The nitrogen coordination present in the complex was affirmed by the peak at 2385 cm- 1. The presence of NH3, C- H and O-H bending vibrations were confirmed by the peaks obtained at 1508 cm-1 and 1381cm- 1. The peaks at 1079cm-1 gave evidence for the presence of C-N stretching and ClO4 stretching vibrations in the μ-peroxo complex [10-12]. |
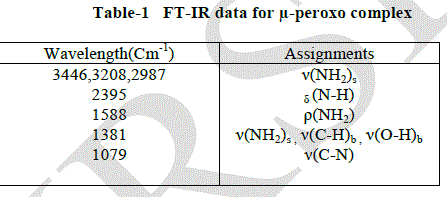 |
| All the kinetic runs were conceded out with [D-ribose] always ten times in excess of μ-peroxo complex. The oxidation of D-ribose was carried out with different initial concentration of μ-peroxo complex. The rate constants were increased by increasing the initial concentration of the complex. The reaction is first order with respect to complex. |
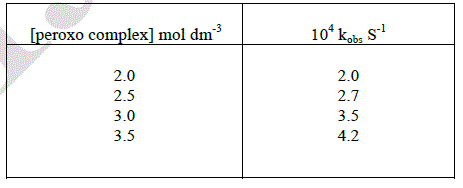 |
| In order to study the effect of [D-ribose], the oxidation of the substrate was carried out with different initial concentration of the substrate. The reaction is zero order with respect to substrate. |
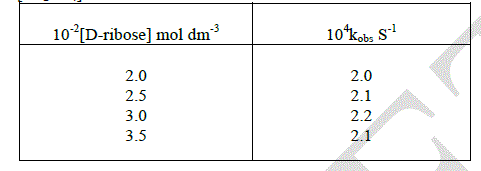 |
| The effect variation of added mineral acid concentration on the rate oxidation was studied by changing the concentration of added Sulphuric acid. The constant were found to be increased by increasing the hydrogen ion concentration. Hence the order with respect to hydrogen ion concentration was found to be one. |
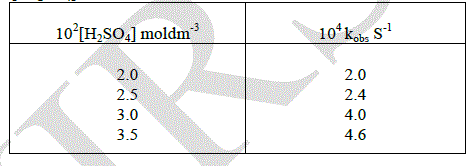 |
| The effect of disparity of added salt on the rate of oxidation of D-ribose was studied by varying the concentration of added sodium sulphate. The rate of the reaction was virtually constant with increasing the initial concentration of added salt of the medium. This shows that the ionic strength is negligible on the reaction rate. |
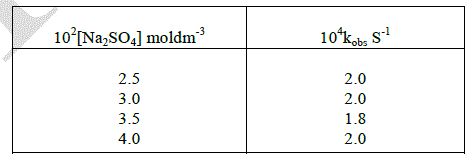 |
| The oxidation of D-ribose was carried out with different temperature. The rate constants were increased by increasing the temperature. |
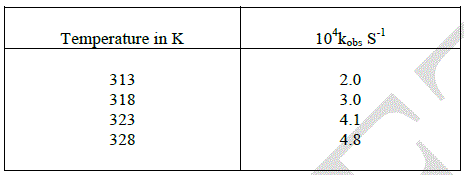 |
| The Arrhenius plot of log Kobs versus 1/T and the plot of log (K/T) vs 1/T gave a straight line with a very fine correlation. The energy of activation, enthalpy of activation, free energy of activation, entropy of activation and A were found to be 49.41 KJmol-1, 46.81 KJmol-1, 79.11 KJmol-1,-103.17 KJmol-1and 2.038 at 313k respectively. The positive value of free energy of activation ΔG* and the enthalpy of activation designate that the transition state is extremely solvated while the negative entropy of activation ΔS* suggests the formation of an activated complex with a reduction in the degree of freedom of molecules. It also suggests the compactness of the transition state as compared to the ground state and also in cyclic nature [13]. |
| Mechanism and Rate law |
| In general, the mechanism of oxidation reactions [14] may be divided into three steps. Activation of molecular oxygen is the first step. The second step is the formation of reactive intermediate. The final step is the Trans formation of intermediate into product is the final step. From the experimental results the most appropriate mechanism for the oxidation behavior of μ-peroxo complex is given as |
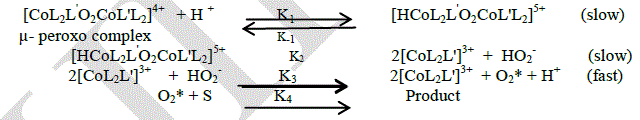 |
| At acidic condition the first step involves the formation of [CoL2L'O2CoL'L2]5+ complex ion from the peroxo complex and in this step there is equilibrium is obtained between the [CoL2L'O2CoL'L2]5+ and the peroxo complex. in the second step the [CoL2L'O2CoL'L2]5+ complex ion dissipate int Co(III) complex and HO2 - ion. The products formed in the second step release activated oxygen with the concurrent reduction of mononuclear Co(III) complex to mononuclear Co(II) complex. This step supported by the mechanism which gives release of oxygen from a μ-peroxo complex Co(III) dimer[15]. The activated oxygen reacts with the substrate and gives the product in the final step. The above mechanism leads to the following rate law, |
| Rate = Kobs [μ-peroxo complex] [H+] |
| This rate law explains all the observed experimental facts. |
References |
|