ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
H. Bandekar1 and S. S. Lele2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This study reports in vitro production of flavonol quercetin from Ficus benghalensis L. which is the first study of its kind to be reported. Different media were employed for callus induction using explants like nodal segments, young leaf discs and root sections. Friable calli were obtained in media supplemented with plant growth regulators (PGRs) after 14-18 days of inoculation. The callus was subcultured and allowed to mature to investigate its biomass accumulation and quercetin production. Proliferation of callus was achieved by supplementing sucrose (3%) Lglutamine (150 mg/L). Liquid phase cultures were established with the abovementioned medium composition without solidifying agents Quercetin was extracted from cultures by fractionation, confirmed by High Performance Liquid Chromatography and quantified using High Performance Thin Layer Chromatography. A maximum quercetin content of 0.31 mg/g dry cell weight (DCW) was obtained through this system which was 20 times higher than that extracted from mother plant.
Keywords |
| Ficus benghalensis L., callus induction, liquid phase cultures, proliferation, quercetin. |
INTRODUCTION |
| Ficus benghalensis L. (Banyan tree) is a woody plant belonging to the fig family Moraceae. This plant is the national tree of India. It has a long life span and has been attributed to attain large dimensions. A unique feature of some species from this genus including Ficus benghalensis is that they have aerial roots systems extending to the ground eventually forming their branches. It is cultivated widely in the tropics and naturalized in almost every wet tropical area. This plant is indigenous to the Asia-Pacific subcontinent and has a wide distribution all over India, also being a native to Myanmar (Burma), Thailand, southern China, and Malaysia [1]. Its global distribution includes Miami, Florida, Queensland, Northern Mariana Islands, Guam, Fiji, French Polynesia [2]. Since time immemorial, several members of this genus Ficus have been used traditionally in a wide variety of ethno medical remedies and have proven to be of great importance. |
| Biomolecules from Ficus benghalensis plants have attracted great attention mainly because of their important role in disease prevention. These plants especially have been known to possess antioxidants like flavonoids, flavonols, tannins etc. which play a major role in scavenging free radicals known to be harmful to the human body, thus acquiring nutraceutical and pharmaceutical importance. Quercetin is a flavonol, a potent anti-oxidant which has been attributed with many properties: anti-cancerous, anti-bacterial, anti-viral, anti-inflammatory, anti-pyretic and anti-diarrheal [3, 4, 5]. |
RELATED WORK |
| The traditional systems of medicine highly rely on various plant parts, in order to obtain the desired extracts for treatment purposes. This demands an invariable dependence on the plant which may lead to overexploitation of natural populations. Also, a large-scale requirement of these important biomolecules may possess a serious threat to the survival of the plant source. Plant tissue culture systems act as efficient alternative tools for in vitro culturing and manipulation of plants to facilitate the production of commercially valuable secondary metabolites in large quantities without any evident harm to the mother plant. In addition, plant cell cultures offer advantages of being able to obtain metabolites which are predictable, easy to isolate than from the complex whole plant, avoiding the interfering intermediary compounds present in whole plants and presenting a potential arrangement for studying elicitation response [6]. As these biomolecules are often accumulated in relatively low quantities in specified plant species, the emerging demand in today's marketplace for natural, renewable products has refocused our attention on in vitro cell cultures as potential factories for secondary plant metabolites. |
| In the present study, we have attempted to develop a plant tissue culture system using explants from Ficus benghalensis for the production a potent flavonol quercetin. However, woody plants have been found to be in general recalcitrant to in vitro regeneration. Hence, various parameters had to be intricately considered while attempting in vitro culture of such plants. In vitro culturing using explants of Ficus benghalensis with the objective of micro-propagation has been attempted before [7, 8]. However, this is the first attempt to obtain secondary metabolite (quercetin) production through tissue culture. Such studies may form the basis for future work of production of other important bioactive compounds from the same or related species through maintenance of tissue culture systems. |
MATERIALS AND METHOD |
Explant preparation and inoculation |
| Nodal segments, young leaf discs and aerial root sections of Ficus benghalensis plants were collected from Institute of Chemical Technology (ICT), Mumbai. Voucher specimens (LMPF1, LMPF2 and LMPF3) were deposited at Medicinal natural product research laboratory, Department of Pharmaceutical sciences, ICT. The study of callus induction from nodal explants and leaf discs was performed earlier in our laboratory [9]. In the present study, aerial roots were also used as explants. An additional step of studying the sterilisation profile for each explant was performed (Table 1). The sterilized explants were transferred to a sterile petri plate and blotted on sterile filter paper. The nodal segments were excised and used for inoculation into the media. |
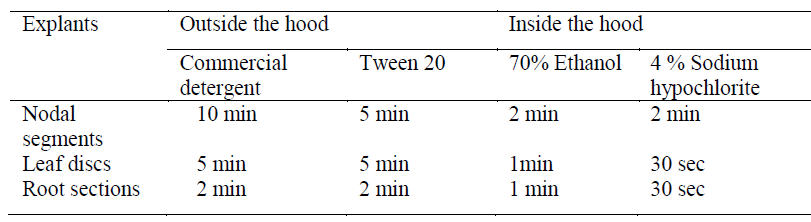 |
Media optimisation |
| Woody plant medium (WPM) [10], Murashige and Skoog (MS) [11] and Gamborg’s B5 medium [12] were used for inoculation of explants. Different combinations of auxins and cytokines were added to the media to study callus induction. According to our previous study on plant growth regulators (PGR), a combination of 2 mg/L Benzyl aminopurine (BAP) and 0.1 mg/L Napthalene acetic acid (NAA) was optimised for callus induction with MS medium [9]. The same PGR combination was used with WPM and B5 media for callus induction. |
Callus proliferation |
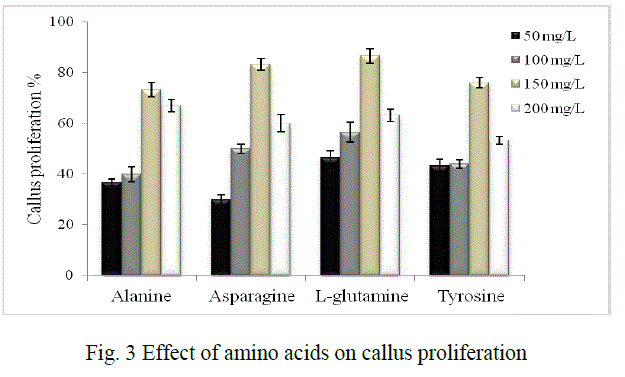
| It was investigated using different carbon sources namely sucrose, glucose, fructose and maltose in concentrations of 2%, 3% and 4% each. Amino acids like alanine, asparagine, L-glutamine and tyrosine in the concentrations of 50 mg/L, 100 mg/L, 150 mg/L and 200 mg/L each were used to study their effect on proliferation. 0.4% bacteriological agar, 0.1% gelrite (Gelrite® Gelan gum, Sigma) were used for solid phase cultures. The pH of the media was adjusted to 5.8±0.2 before autoclaving. |
| Incubation conditions: The culture tubes were incubated in 16 h light/8 h dark photoperiod for callusing and proliferation in a plant growth chamber. |
Cell suspension cultures |
| Cell suspension cultures were initiated from calli in liquid media of same medium composition as that of solid agar medium without the solidifying agents. The cultures were kept in orbital shaker maintained at 100 rpm at 25 ºC and 16 h light/8 h dark photoperiod. Henceforth, the stock suspension culture was subcultured after every 18 days to fresh medium in a ratio of 1:1. The remaining of the culture was used for various analyses. |
Growth measurements and viability testing |
| Fresh cell weight (FCW) and dry cell weights (DCW): The experiments were performed in non-sterile conditions taking 2ml of the sample every day for a week. FCW were estimated collecting the cell suspension using 2 mL Eppendorf tube and repeatedly washing the cell pack with about same volume of distilled water. The DCW was determined after drying the cells in at 50°C overnight. |
| Settled cell volume (SCV) and packed cell volume (PCV) from cell suspension cultures: Both parameters were chosen for fast estimation of culture growth in vitro. Settled cell volume was determined by allowing 10 mL of cell suspension to sediment in pre-autoclaved 15 mL graduated conical centrifuge tubes after 30 min intervals for 4 h. It was calculated as the percentage of the volume of sediment cell mass to total volume of suspension. On the other hand, the PCV were determined in terms of the volume of packed cell after centrifuging at 1000 rpm for 10 min to the total volume of the suspension. |
| Cell viability counting: Viability of the cells was checked using fluorescien diacetate (FDA) counter stained with Evans Blue (EB) test every 10 days at the time of sub culturing. 1 mL the suspended sample was stained with 10μL of FDA stock and incubated in a complete dark chamber for 15 min in an Eppendorf tube. The sample was then centrifuged at 1000 rpm for 10min and the pellet was resuspended in 1mL of distilled water for 3 min. Counter staining was performed using 100 μL of 1% Evans Blue for 10 min at room temperature [13]. Thereafter, random counts for cell viability were done by means of a haemocytometer under microscope. |
Extraction and analysis of bioactive compound of interest (quercetin) |
| Extraction of quercetin from plant parts (leaves) and cell cultures (cell biomass): Leaves of the plant and cell biomass from laboratory cell cultures were dried overnight at 50º C. The dried samples (20 g) were extracted in 100 mL methanol for 2 h using Soxhlet apparatus. The methanolic extract was concentrated using a rotary evaporator to dryness. The dried extract (1.3 g) was then subjected to hydrolysis by refluxing with 100 mL of 7% H2SO4 for 30 min. The reaction mixture was filtered and the filtrate was extracted with 2 portions of ethyl acetate (25 mL) in a separating funnel. The ethyl acetate layer was combined, washed with distilled water and dried. The residues for leaf sample (47 mg) and cell biomass sample (52 mg) were obtained of which 5mg of each sample was dissolved in 10 mL methanol and 100 μL was spotted for High Performance Thin Layer Chromatography (HPTLC) analysis. |
| Instrumentation and chromatographic conditions: Thin layer chromatography was performed on 20 cm × 10 cm HPTLC silica gel G60F254 (Merck, India). Standard solutions and samples were applied at 15 mm from the base of the plate using Camag Linomat 5 automated spray-on applicator equipped with 100 μl (Hamilton, USA) syringe. The plates were developed to a distance of 80 mm in mobile phase Toluene: Ethyl acetate: Acetone: formic acid (5: 2.5: 7.5: 0.5 v/v) at room temperature (28 + 2º C) in a Camag glass twin-through chamber previously saturated mobile phase vapour for 20 min. After development, the plates were air dried. The chromatogram was obtained after densitometric scanning at 254 nm with a Camag TLC scanner 3 controlled under Camag winCATS planar chromatography software. |
| Standard curve and quantification of quercetin: A stock solution of standard quercetin (1 mg/mL) was prepared in methanol and diluted with methanol (1:100). Different volume of stock solution 1, 2, 3, 4, 5 and 6μL, were spotted on to TLC plate to obtain concentration 10, 20, 30, 40, 50, 60 ng /spot of quercetin respectively. The amount of quercetin in the leaf and cell biomass samples was then determined (mg/g dry cell weight basis) from the standard curve. |
| Isolation and High Performance Liquid Chromatography (HPLC) analysis of quercetin: 100 g of dried cell biomass was subjected to extraction procedure mentioned above. The ethyl acetate layer so obtained was dried and loaded on a silica gel column using eluent chloroform: methanol (90:10) gradually increasing to (40:60), at which quercetin eluted. In order to ascertain the purity of the isolated quercetin obtained from cell cultures, HPLC analysis was performed with a JASCO (Hachioji, Tokyo, Japan) system, using 250 mm x 4.6 mm i.d., RP-18 (5-μm particle size) Purospher star column (Merck), with a flow rate of 1.00 mL/min and mobile phase methanol : water (60:40) at 254 nm. |
| All chemicals, unless mentioned, were of analytical grade and procured from HiMedia, India |
RESULTS AND DUSCUSSION |
| Callus induction, callus proliferation and cell suspension cultures |
| Callus induction: Explant screening was done using nodal segments, young leaf discs and root sections. Callus induction was observed after 14-18 days of inoculation. Depending on the medium used, maximum callusing was observed when nodal segments were used as explants in MS medium (Fig. 1). Callusing depends on an intricate interplay of factors like sterilisation processes which reduce major contaminants including viruses, bacteria, yeast, and fungi and the medium constituents, thereby rendering explants screening an essential step for obtaining good calli. Ethanol was used in our study singly and at ranges of concentration and various exposure times. Thereafter, hypochlorite was selected and evaluated for its sterilisation effect. The dissociation of hypochlorite salts NaOCl in water to form HOCl has been reported as its significant effect for such activity [14] .In our study we found that 70% ethanol in less than 2 min exposure followed by 4% sodium hypochlorite (2 min) is effective for nodal segments sterilization. The young leaves and root sections however, required about 1 min, 70% ethanol and 30 sec hypochlorite exposure (Table 1) Such ethanol-hypochlorite combination was found to be effective to get >80% viable explants and taken as optimum condition for further studies. At higher concentrations and higher exposure time, toxicity was observed. |
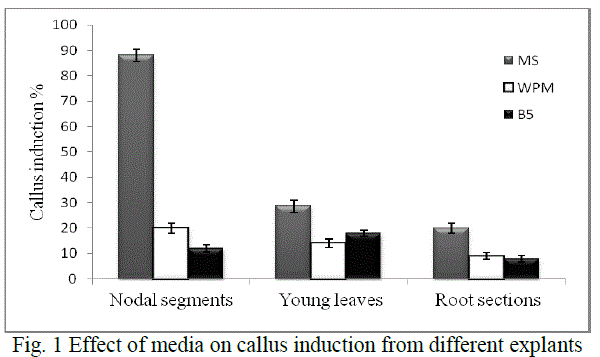 |
| MS medium with growth regulators was shown to induce callus better in all the explants than growth regulators supplemented WPM and B5 media (Fig. 1). The variations of callus induction on different media may be due to the differences of NO3 -/NH4 + ratio, an important factor on nitrogen uptake and pH regulation during plant tissue culture with is in agreement with a previous study [15]. The effect of growth regulators is significant in plant cell and tissue culture practices. Hence various growth regulators singly or in combination were evaluated for induction and development of callus. |
| Hence, nodal segments were used as explants in MS medium with 2 mg/L BAP and 0.1 mg/L NAA for callus induction in all experiments further carried out. Callus maturation was done on the medium optimised for 4 weeks - 2 months followed by successful subculturing to fresh media. |
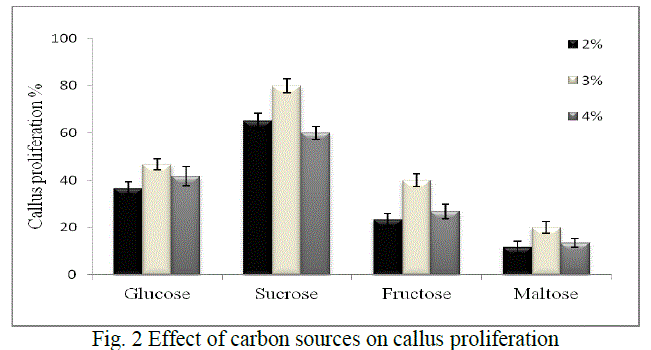
| Callus proliferation: Low carbon dioxide and light supplied in vitro are the main reasons that cultures require sugars for growth especially in liquid phase culture system. The cultures are not able to grow properly without an exogenous supply of carbohydrates [16, 17, 18]. Hence the effect of various carbon sources which includes glucose, sucrose, fructose and maltose were evaluated to improve callus proliferation in our study. Sucrose is used in plant cell culture for various purposes either as a carbon source or as a regulator of cell osmosis. The range selected was 2%, 3% and 4% of each sugar, as studies have shown that high sugar levels might also cause cell dehydration and affect cell proliferation [19, 20]. Glucose has been reported as an important additive for callus induction and embryogenic callus formation [21, 22] although it was found to be less suitable than sucrose in our study. Also, maltose has been known to show equal or more efficiency than sucrose in supporting embryogenesis in a number of species [23, 24] but in our studies it supported the least proliferation. Fructose has been also reported to support callus induction and proliferation of Bougainvillea sp. over sucrose or glucose [25] but our studies did not support this. Hence, in our study maximum callus induction obtained was 80% when sucrose (3%) was added as a sugar source (Fig. 2) |
 |
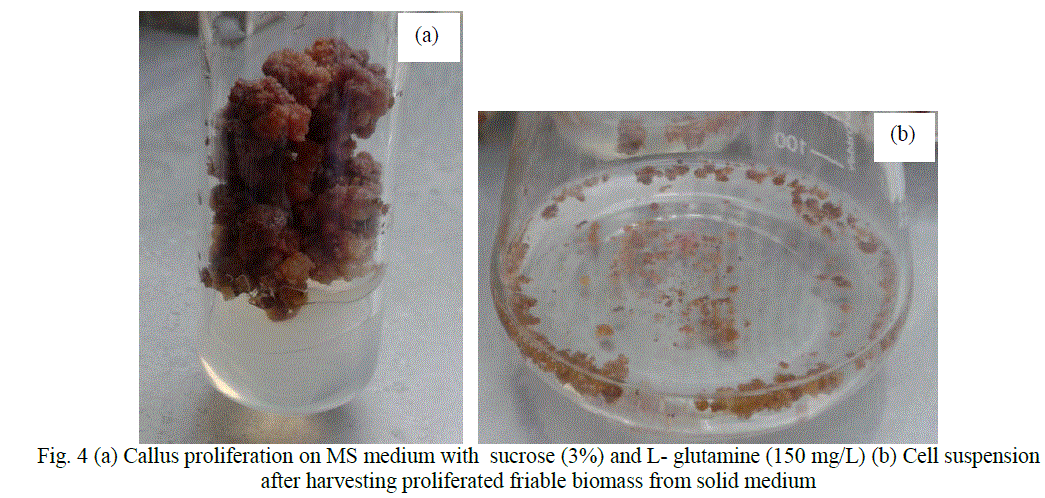
| Figure 4 (a) shows proliferated mass of cells on MS medium supplemented with 3% sucrose and 150 mg/L L-glutamine. |
| Cell suspension cultures were established in MS medium with 3% sucrose and 150 mg/ L L- glutamine (Fig. 4b). Cell viability testing results showed 80% viable cells during the time of subculture and experimentation. |
 |
| The quantification of quercetin from samples was done using standard curve. A good linearity was observed in standard curve with R2= 0.9999 in the said concentrations (Fig. 5b). From the standard curve the concentrations of quercetin in leaf sample obtained from the mother plant was found to be 0.018 mg/g which lies in good agreement with previous study (Taskeen et al. 2009). Quercetin from the cultured cell biomass obtained through plant tissue culture was found to be 0.31 mg/g dry cell weight basis. From this result, it is seen that quercetin from cell biomass cultured in the laboratory amounted to 20 times than that obtained from the mother plant which is a very significant increase suggesting the commercial importance of this system. |
| Isolation of quercetin and HPLC analysis |
 |
| Fig. 6 HPLC chromatogram of (a) purified quercetin fraction collected from silica gel chromatography (a) ethyl acetate extract from cell biomass |
| Isolation of quercetin from cultured cell biomass was done using silica gel chromatography that afforded 32 mg of the compound, which lies in good agreement with the HPTLC results. The purity of the isolated quercetin was ascertained by HPLC and was found to be > 98% w/w. The chromatogram of the isolated compound from cell biomass and chromatogram of the ethyl acetate extract obtained from cell biomass is shown in Fig. 6. |
CONCLUSION |
| With successful production of quercetin through cell cultures of Ficus benghalensis, this research work promises an alternate source system of commercially important bioactive compounds. Future scope involves maintenance of high quercetin producing cell lines of Ficus benghalensis should be cultivated in large scale bioreactor for major production after studying the feasibility parameters. |
ACKNOWLEDGEMENT |
| The authors are thankful to University Grants Commission- Special Assistance Program (UGC-SAP), India for providing the financial support for the project. |
References |
|