ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Samy M. El-Megharbela,b*, Ahmed El-Maghrabya,c and Moamen S. Refata*,d
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The carbonate slats of Ca(II), Sr(II), and Ba(II), respectively, were synthesized by a new simple and cheap materials via the chemical reaction of an aqueous solutions of metal chloride ions (CaCl2, SrCl2 and BaCl2), potassium iodide and urea with molar ratio 1:2:10, respectively, at ~ 100 oC for 3 hrs. The infrared spectra of the results indicate absence of the essential bands of urea, but existed of the characteristic bands of ionic carbonate, CO3 2–. An important chemical mechanisms discussed the preparation of Ca(II), Sr(II), and Ba(II) carbonate compounds were suggested
Keywords |
| carbonate CO3 2–, infrared spectra, alkaline earth metals, potassium iodide, urea. |
INTRODUCTION |
| Urea is physiologically very important. It is the chief nitrogenous product of protein metabolism. Urea has a melting point of 132ïÃâðC, soluble in water and ethanol, but insoluble in ether. Urea is used for preparing formaldehyde- Urea resin (plastics) [1], barbiturates [2], and fertilizers [3-6]. Urea is also extensively used in the paper industry to soften cellulose and has been used to promote healing in infected wounds and many other applications in the field of biological and organic synthesis [7-10]. Yamaguchi and Stewart [11, 12] were assigned all of the observed frequencies in the spectra of urea and urea-d4. The two vibrations of the frequencies at 1686 and 1603cm-1 were assigned as the 1686 cm-1 band due to CO stretching vibration and the 1603 cm-1 band for NH2 bending motion. The calculations studied by Yamaguchi showed that for the band at 1686 cm-1, the contribution of the NH2 bending motion is greater than that of CO stretching motion. The infrared bands of urea-d4 observed at 1245 and 1154 cm-1 are assigned to ND2 bending vibrations. This assignment is consistent with the observed depolarization degrees of the Raman lines. The 1464cm-1 frequency of urea is assigned to the CN stretching vibration. The corresponding frequency of urea-d4 is observed at 1490cm-1. The 1150cm-1 band is assigned to NH2 rocking vibrations. The reactions between transition metal ions and urea at room temperature have been studied extensively [13-17]. The infrared spectra of these complexes clearly indicated that urea molecule behaves as a mono dentate ligand and coordinates to the metal ions through the oxygen atom and not the nitrogen atom. The nature of the reaction products depend strongly on the type of metal ions and so the metal salt used. The novelty in our previously studies [18-27] were oriented to the reaction of urea ligand with different metals such as Co(II), pb(II), Sn(II), Cr(III), Fe(III), Au(III), Sn(IV), V(V) and Mo(IV) at high temperature which demonstrate that the types of metal ions beside their anions have a pronounced effect on the nature of the reaction products. The published papers were trended for the reaction of urea with different metal salts at elevated temperature lead to discovering a novel method for preparation pbCO3 and CoCO3 [21], lanthanide carbonates [23,27], limonite, FeO(OH) [20], 2ZnCO3.3Zn(OH)2 [19], SnOCl2.2H2O [18], (Cr2O3, MnO2, MoO3 and WO3) oxides resulted from a novel oxidation reduction reaction between (K2CrO4 or K2Cr2O7), KMnO4, Na2MoO4 and Na2WO4, respectively, with urea in an aqueous solution at ~ 85 oC [27]. The aim of this paper was focused to identify the nature of the reaction mechanisms of the products resulted during the reaction of (CaCl2, SrCl2 and BaCl2), potassium iodide and urea with molar ratio 1:2:10, respectively, at ~ 100 oC for 3 hrs in aqueous media. The reaction products were isolated as solids and characterized by infrared spectroscopy technique. |
II. EXPERIMENTAL |
| All chemicals used throughout this work were analytical pure. MCO3 (M = Ca(II), Sr(II) or Ba(II)) were prepared by mixing an aqueous solutions (100 ml) of 0.1M of urea, 0.02 of potassium iodide with 0.01M of the respective CaCl2, SrCl2 and BaCl2. The mixtures were heated at 100 oC for 3 hrs in a hot plate. The solid products compounds were filtered off, washed several times with hot water, dried at 100 oC in an oven for 3 hours and then placed in vacuo over anhydrous calcium chloride. The yields of the obtained Ca(II), Sr(II) and Ba(II) carbonates were varied in the range 75-to-80% depending upon the type of metal as well as on the counter ions associated with the metal ion. Carbonate content in the four compounds were determined by dissolving a sample of each product in excess standard HCl and the excess of HCl was determined using standard sodium carbonate [28]. The percentage of calcium(II), strontium(II), and barium(II) in their compounds were determined gravimetrically method till constant weight and stable formula. The infrared spectra of urea, all reactants and products were recorded using a Bruker FT-IR Spectrophotometer. |
III. RESULTS AND DISCUSSION |
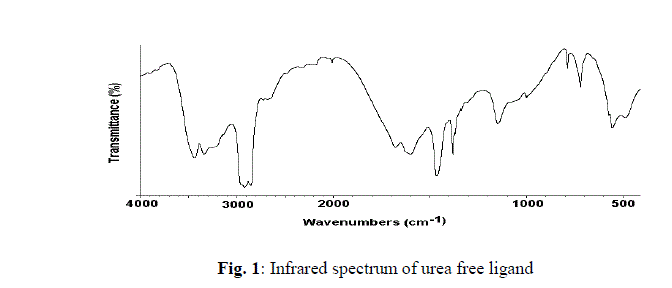
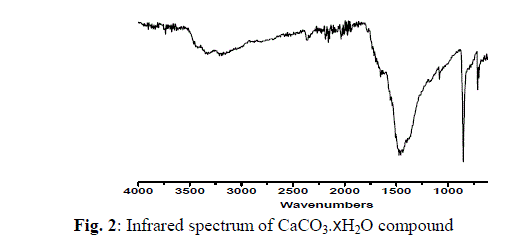
| The chemical reactions of 0.1M of urea, 0.02 of potassium iodide with 0.01M of the respective CaCl2, SrCl2 and BaCl2 with molar ratio (10: 2: 1), respectively, at 100 o C produces a white fine solid powders. The infrared spectra of urea as well as the reaction products of different CaCl2, SrCl2 and BaCl2 salts with urea at boiling point of water were obtained. The spectra of free urea ligand, calcium(II), strontium(II) and barium(II) carbonates are shown in Fig. 1-4, respectively. The band assignments for the products are given in Table 1. The infrared spectra show no bands due to any of the reactants and of coordinated urea, but instead, a group of bands characteristic for the ionic carbonate, (CO3)2-, is appeared [29]. Based on this fact, along with that obtained data from the volumetric determination of (CO3)2- group with standard solution of HCl and beside that the infrared spectra of the commercially obtained CaCO3, SrCO3 and BaCO3 are the same as that of the reaction products. The products obtained were identified as CaCO3, SrCO3 and BaCO3. The infrared assignments agree quite well with those known [29] for the ionic carbonate (CO3)2-. The inhibiting action of amides and alkaline earth element acetates on the corrosion of metals gives a practical importance to the investigation of their reaction in aqueous solutions. The reaction of urea with alkaline-earth metals in aqueous solutions has been studied by a number of workers [30-34]. In case of CO(NH2)2-M(CH3COO)- H2O (M=Mg, Ca, Sr or Ba) systems [35,36], the tendency of the alkaline earth elements to form complexes with urea decreases as its ionic radius increases. Thus magnesium(II) forms a complex with two molecules of urea, calcium(II) forms a complex with one molecule of urea, while strontium and barium acetates don’t react. The complexes [2CO(NH2)2.Mg(CH3COO)2] and [CO(NH2)2.Ca(CH3COO)2], are identified by elemental, X-ray phase, thermogravimetric analysis. The system MgCl2-CO(NH2)2-H2O has been studied [30]. Its solubility isotherm at OïÃâð consists of three branches. The two branches on the outside correspond to the crystalline of urea and MgCl2.6H2O, while the middle branch corresponds to MgCl2.4CO(NH2)2. With a further temperature rise to 15ïÃâð, the hydrated complex MgCl2.CO(NH2)2.4H2O is formed. At 30ïÃâð another complex, [MgCl2.6CO(NH2)2] is formed. All of these complexes are congruently soluble and are precipitated in crystalline forms. At 45ïÃâð the complex [MgCl2.10CO(NH2)2] is formed. The bond between MgCl2 and urea is formed through the oxygen of the carbonyl group, as indicated by infrared spectroscopy. The role of M(II) (Ca, Sr or Ba(II)) ions in decomposing the coordinated urea at high temperature may be understood as follows. |
 |
 |
 |
References |
|