ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Sekar1, R. Manikandan2, A. Mandal3, S. Sankar4, T.P. Sastry*5
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Recently development of nanobiocomposites (NBC) from biowastes, which has potential biomedical applications has gained momentum. Extraction of collagen for biomedical applications from biowastes such as fish scales has received much attention. The present study aims at designing a biocompatible collagen based NBC impregnated with silver nanorods (AgNRs) and coupled with ciprofloxacin to make it suitable for biomedical purposes. Collagen coated AgNRs were graft co-polymerized with a biocompatible polymer, glycidyl methacrylate (GMA), and coupled with an antibiotic, ciprofloxacin (C-Ag-G-D). Infrared spectroscopy studies confirmed the grafting of GMA on C while SEM images showed coating of collagen on silver nanorods. DSC and TGA results indicate higher denaturation temperature with improved thermal stability for the NBC. Antimicrobial studies using E.Coli have shown enhanced antibacterial effect for the NBC. Thus, C-Ag-G-D nanobiocomposite with improved thermal stability and antibacterial activity might be a suitable candidate for biomedical applications.
Keywords |
| Fish scales, Collagen, Silver nanorods, Nanobiocomposites, Ciprofloxacin. |
INTRODUCTION |
| Collagen, a biopolymer is a major constituent in the connective tissue proteins of vertebrates. Among the 14 different kinds, type I collagen is the most common. Its use as a biomaterial in various forms such as sutures, wound dressing agents, haemostatic agents, surgical tampons, vascular prosthesis etc is well established [1]. Collagen in pure form has low immunological activity and thereby acts as an excellent substrate for cell attachment and cell in growth [2-4]. The advantages of using a naturally-derived material such as collagen arise from its biocompatibility and intrinsic biological recognition [5-8]. This biopolymer possess features such as high biocompatibility and biodegradability, low toxicity and immunogenicity compared to other natural polymers. Also, its structure and interaction with host tissue makes it an efficient matrix to produce diverse forms of biomaterials and therefore used extensively in various biomedical fields such as scaffold in tissue engineering applications [9]. Although synthetic polymers have been widely used as a biodegradable material in tissue engineering, bio-derived materials such as collagen are being increasingly applied in regenerative medicine. Type I collagen extracted from bovine source is mainly used in biomedical applications. However, recent reports of several transmissible diseases such as bovine spongiform encephalopathy etc observed in collagen products extracted from bovine source has further led to the search for safer alternative options [10, 11]. Thus, collagen extracted from bio wastes such as fish scales have gained significant importance and received attention. Silver nanoparticles have been studied for many years not only for their antibacterial activity but also for their low toxicity [12-16]. They are small in size and possess high surface area which enables them to effectively fuse with bacterial cell membranes and thereby serving as an effective antibacterial agent [17]. Multifunctional materials, containing silver nanoparticles in reactive or non reactive polymer networks have attained top priority in research as biocidal products, biomaterials, drug delivery vehicle etc for biomedical applications [18, 19]. Recently, research is focused on the third generation of biomaterials such as composites and nanocomposites which have enhanced bioactive and bioresorbable properties. Thus, the combination of the properties of collagen and silver nanorods could create a unique biomaterial with improved and novel characteristics. Ciprofloxacin (1-cyclopropyl–6–fluro–1,4–dihydro–4–oxo–7–[1–piperaZinyl]–3–quinoline carboxylic acid hydrochloride monohydrate) is the drug of choice as it exhibits broad spectrum antimicrobial activity and shows bactericidal effects on gram negative bacterial strains. In addition to that, it has good tissue penetration property essential for the closure of wounds at the site of infection [20]. Therefore, the objective of this study is to devise a ciprofloxacin entrapped collagen based NBC containing silver nanorods with improved properties which could be utilised for biomedical applications. The schematic representation of this study is shown in Fig. S1. |
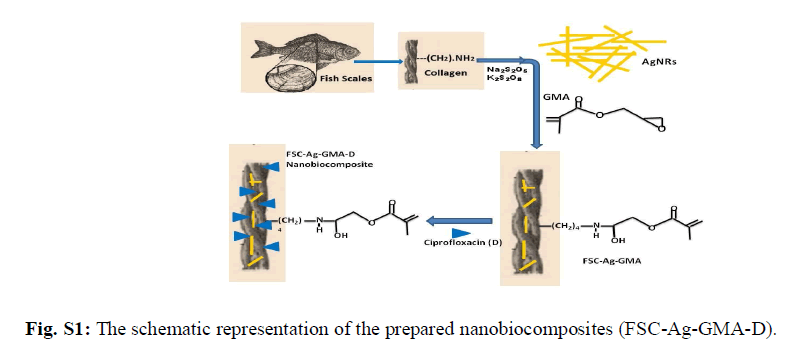 |
II. MATERIALS AND METHODS |
| Fish scales were collected from nearby fish market. Glycidyl Methacrylate (GMA), silver nitrate (AgNO3), sodium borohydride (NaBH4) was purchased from Sigma-Aldrich, St. Louis, Mo, USA. Bacterial strain, Escherichia coli (E. coli) was kindly supplied by the Microbiology Department of the Central Leather Research Institute, Chennai. Muller Hinton medium was purchased from Hi-media laboratories Pvt. Ltd, Mumbai, India. All other reagents used were of analytical grade. a) Preparation of fish scale collagen The isolation of collagen from fish scales was performed using a modified method reported by Sankar et al [21]. In brief; fish scales were washed with running water to remove sand and other foreign bodies and later exposed under sunlight. 200 g of dried fish scales were soaked in 10% sulphuric acid solution for 24 h. The fish scales were then minced with an industrial mixie. The resultant fine paste was subjected to centrifugation (12,000 rpm) at 4ºC for 20 mins. This supernatant was collected and its pH was adjusted to 7 using calcium hydroxide solution. The supernant solution was further centrifuged at 10,000 rpm for 15 mins to remove calcium sulphate salts. The supernatant solution contains collagen (60% solids) which was stored at 4oC until further use. b) Preparation of fish scale collagen film (C) To the fish scale collagen (20 mL), ethylene glycol (10 μL) was added and mixed thoroughly. Subsequently, the solution was poured into a polythene tray having a dimension of 10 x 6 cm and air dried at room temperature (30ºC) to obtain the film. c) Preparation of silver nanorods (AgNRs) The experiment was carried in a dark cold room at 4ºC. To 2 mL of AgNO3 (0.001 M) solution, 30 mL of sodium borohydride (0.002 M) solution was added dropwise with continuous stirring for 20 min. The appearance of pale yellow colour indicates the formation of silver nanorods in the solution [22]. d) Preparation of fish scale collagen film impregnated with AgNRs (C-Ag) To the beaker containing 10 mL of the fish scale collagen solution, added 10 mL of silver nanorods solution with continuous stirring by the magnetic stirrer. The process of stirring was carried on for 15 min. Subsequently, 10 μL of ethylene glycol was added to the contents. The resultant solution was then poured into a polythene tray with measurements of 10 x 6 cm and air dried (30ºC). This dried film was stored in a polythene cover. e) Preparation of C-Ag graft copolymerized with GMA (C-Ag-G) To a beaker containing 10 mL of the fish scale collagen solution, added 10 mL of silver nanorods solution with continuous stirring by the magnetic stirrer. The process of stirring was carried on for 15 min. 25 mg of potassium per sulphate and 25 mg of sodium meta bi-sulphate were added to the contents followed by the subsequent addition of monomer GMA (100 μL) and stirred for 1 h. Later, 10 μL of ethylene glycol was added to this solution (all the experiments were carried out in N2 atm) and poured into a polythene tray with measurements of 10 x 6 cm and air dried. This dried film was then stored in a polythene cover. f) Incorporation of drug into C-Ag-G film (C-Ag-G-D) To a beaker containing 10 mL of the fish scale collagen solution, added 10 mL of silver nanorods solution with continuous stirring by the magnetic stirrer. The process of stirring was carried on for 15 min. 25 mg of potassium per sulphate and 25 mg of sodium meta bi-sulphate were added to the contents followed by the subsequent addition of monomer GMA (100 μL) and stirred for 1 h. To this solution, 1mg of the ciprofloxacin drug is added and is stirred for 1 h. Finally, 10 μL of ethylene glycol was added to the solution (all the experiments were carried out in N2 atm.) and poured into a polythene tray with measurements of 10 x 6 cm and air dried. This dried film was stored in a polythene cover. |
III. CHARACTERIZATION |
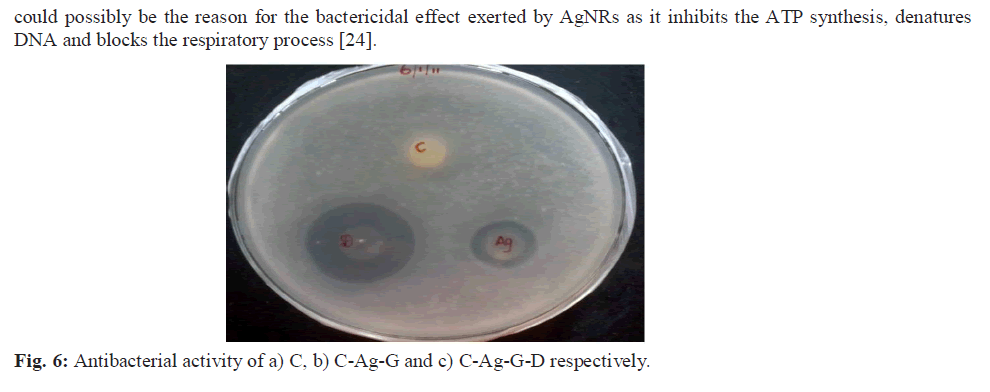
| The NBC prepared was characterized using UV, SDS-PAGE, IR, DSC, TGA, SEM, EDAX techniques and the antibacterial activity was evaluated using Escherichia coli, a Gram negative bacterial strain. a) UV-vis Spectroscopy The UV-Vis absorption spectra were recorded for the samples on a Jasco UV-VIS-V530 spectrometer with a spectral resolution of 2 nm. b) SDS-PAGE SDS-PAGE was done by Laemmli’s method and an 8% gel was prepared [23]. The films were dissolved in SDS sample buffer solution containing 1% SDS, 1% mercaptoethanol, 20% glycerol and heated for 5 min at 100°C. The films were subjected to gel electrophoresis at a constant current of 2 V/cm. After electrophoresis, the gels were stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 and the gel images were captured on a BIOVIS gel documentation system. c) Tensile Strength Two dumbbell-shaped specimens of 4 mm wide and 10 mm length were punched out from the prepared films using a die. Mechanical property, tensile strength (MPa) was measured using a universal testing machine (INSTRON model 1405) at an extension rate of 5 mm/min. d) Fourier Transform Infrared Spectroscopy FTIR spectra of samples prepared were recorded using Nicollet impact 400 FTIR spectroscopy by preparing a 500 mg KBr pellet containing 2-6 mg of the sample. e) Differential Scanning Calorimetry (DSC) DSC of the prepared films was recorded using calorimeter (DSC 204). The heating rate (1K/min) and temperature range between -5ï°C and 200ï°C in an N2 atmosphere, were maintained. f) Thermal gravimetric analysis The thermal stability of prepared samples was determined with a thermo gravimetric (TG) analyzer (Perkin-Elmer TGA) over a temperature range of 37ºC to 585ºC at a heating rate of 20ºC/min under nitrogen atmosphere. g) Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Analysis (EDX) SEM measurements were carried out on a Leica stereo scan-440 scanning electron microscope equipped with phoenix EDX attachment. The EDX spectrum was recorded in the spot profile mode by focusing the electron beam onto the specific regions of the NBC. h) Antibacterial screening To establish the antibacterial properties of the C, C-Ag and C-Ag-G-D, the bacterial cells were cultured aerobically in 25 mL of nutrient broth at 37ºC for 24 h. Disc diffusion method with some modifications was used for screening the antibacterial activity of samples. Nutrient agar (for bacteria) plates were inoculated with 0.1 mL of an appropriate dilution of the tested culture (E. coli, A.T.C.C. 8739). C, C-Ag and C- Ag-G-D discs were placed on the surface of the inoculated plates and incubated at 37ºC for 24 h. The diameter of inhibition zone (mm) including the disc diameter was measured to evaluate the antibacterial activity [24]. |
IV. RESULTS AND DISCUSSIONS |
| a) UV-vis spectroscopy (UV-Vis) The UV absorption maxima of prepared AgNRs dispersed in the sodium chloride solution is recorded where the absorption band was observed in the visible range at 401 cm-1 Fig. 1 (b). The observed absorption band is in agreement with that of the values reported for AgNRs in the literature [22, 25]. |
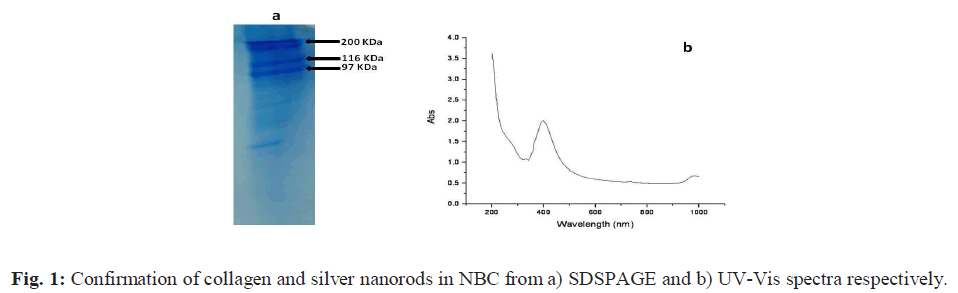 |
| b) SDS-PAGE The result of the SDS-PAGE for collagen isolated from fish scales is depicted in Fig. 1 (a). The characteristic α1, α2 and β bands were observed which confirms the triple helicity of the type I collagen. c) Tensile strength (TS) The tensile strength (TS) of collagen film prepared was found to be 2.1 MPa. The low tensile strength value observed was because the demineralized scales were finely grounded into a paste and reconstituted in the form of a film. This result is in accordance with the findings reported in our earlier study [21]. However, the tensile strength of the C-Ag- G-D film was found to be 4.5 MPa. This significant increase may be due to the co-polymerization of GMA to fish scale collagen. The increased value of tensile strength fulfils the requirement for its usage as a wound dressing material. d) Fourier Transform Infrared spectroscopy (FTIR) The FTIR spectra of the films are given in the Figs. 2 (a to d). The IR spectrum of C shows the characteristic amide absorption bands at 1628, 1535 and 1239 cm-1 representing amide I, amide II and amide III respectively. Also, the bands observed between 1101-1154 cm-1 are assigned as hydroxyl groups present in the collagen. However, collagen in the presence of silver (C-Ag) shows a shift in the absorption peaks of amides which can be attributed to the presence of silver nanorods. In the sample (C-Ag-G) a peak at 1725 cm-1 represents the carbonyl moiety of GMA, the presence of oxirane ring of glycydyl group was observed at 850 and 906 cm-1 respectively. This confirms the grafting of GMA on to C-Ag. The IR spectrum of the drug ciprofloxacin shows the C-C- stretching in aromatic compound at 1507 cm-1, the carboxylic group is represented at 1398 cm-1. The N-H stretching and C-N stretching is represented at 1147 cm-1 and the C-F stretching band is noticed at 1247 cm-1. The IR spectrum of C-Ag-G-D shows a band shift for C-C stretching from 1507 cm-1 to 1548 cm-1 while the peak at 1398 cm-1 is absent. The C-C aromatic shift could possibly be due to the interaction of the drug with the functional groups of collagen. The wide peak around 3417 cm-1 shows the formation of hydrogen bonding; this COO- group might have reacted with the NH2 group available on the backbone of collagen and probably the cause for the absence of COOH peak at 1398 cm-1. |
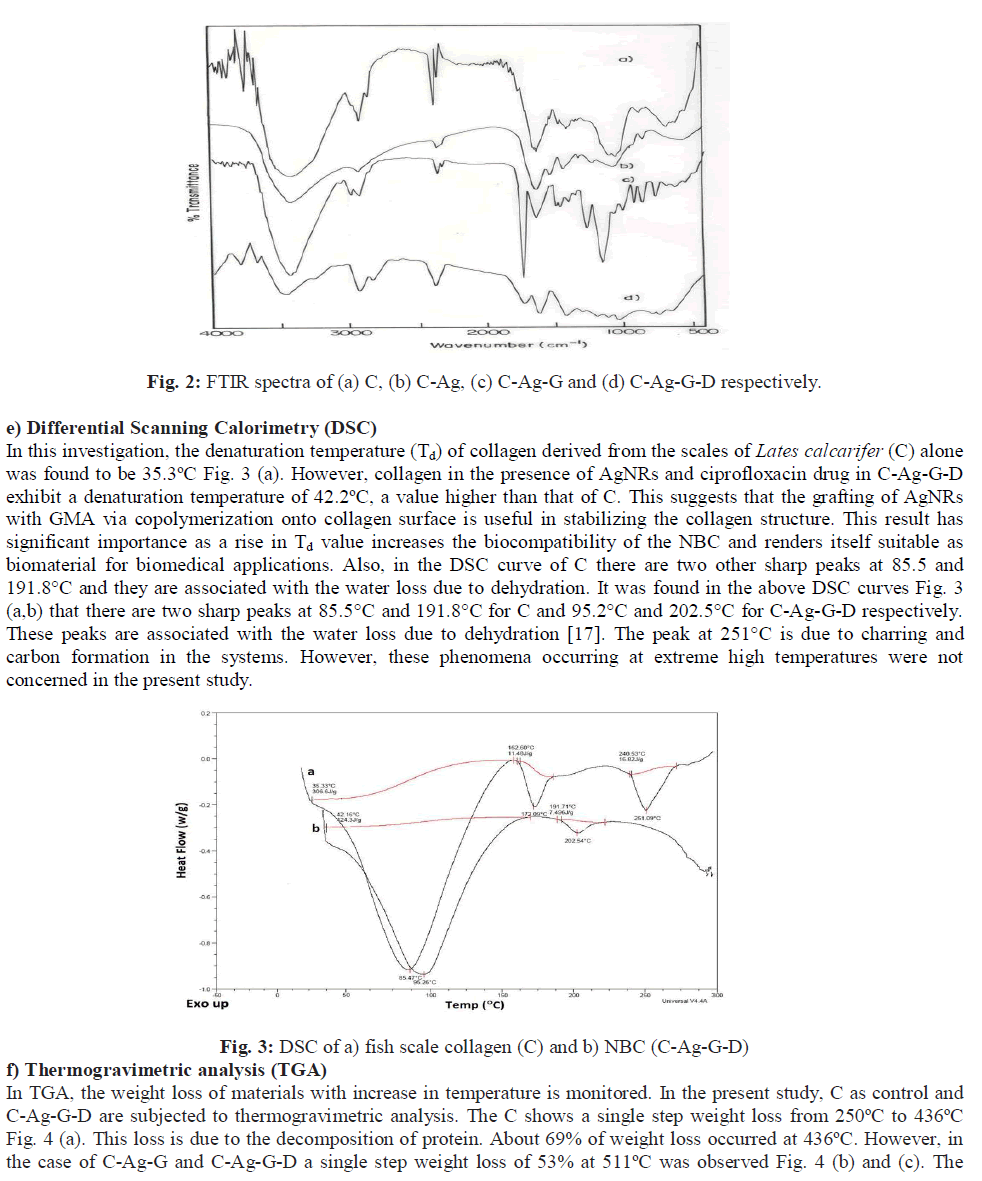 |
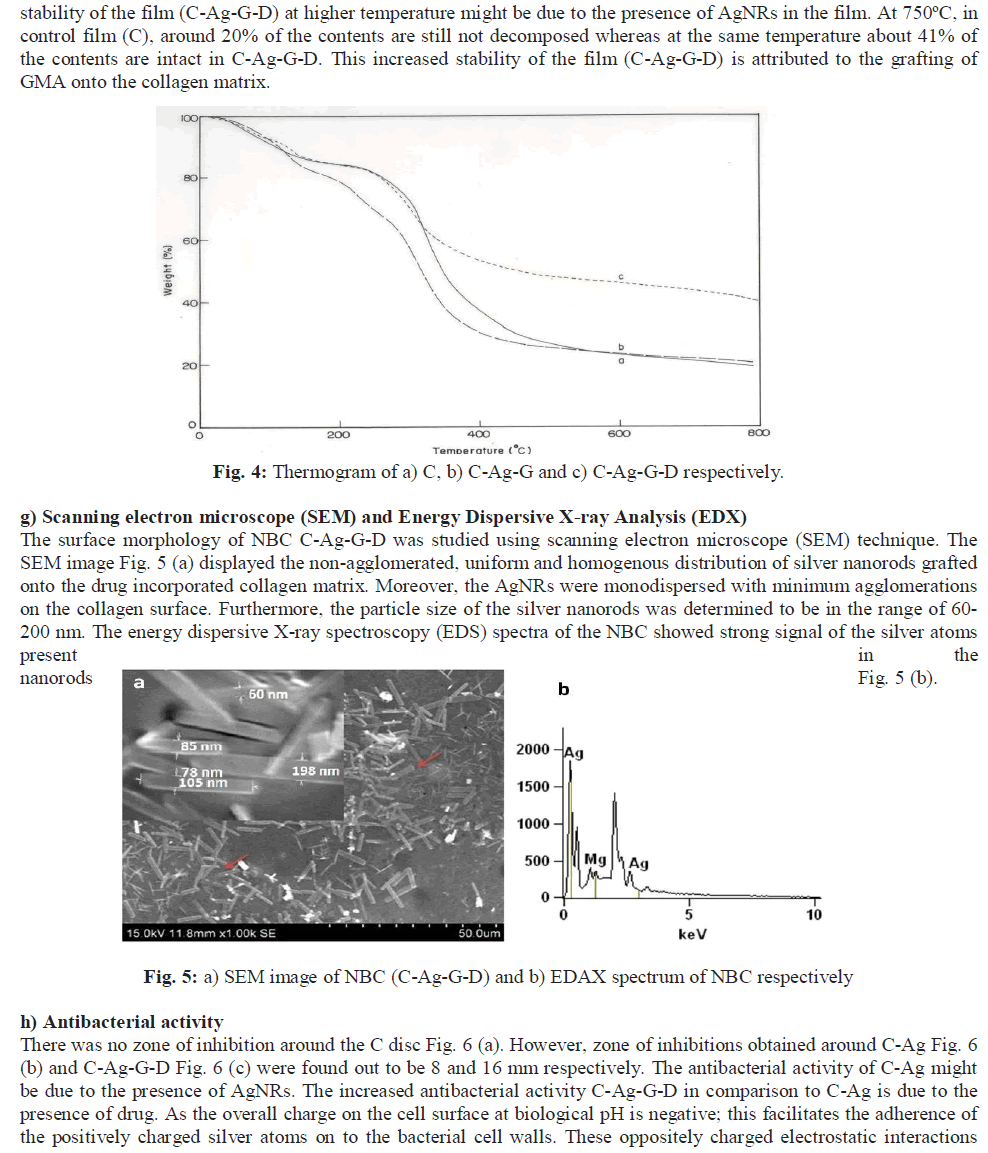 |
 |
V. CONCLUSIONS |
| The NBC C-Ag-G-D possessed a tensile strength of 4.5 MPa, which is better that of C (2.1 MPa). The enhanced antibacterial effect of the NBC was observed on the E. coli, a gram negative bacterial strain. Hence, the association of AgNRs and ciprofloxacin with biopolymer such as collagen derived from fish scales offers an attractive approach in developing biocomposites that has potential in biomedical applications especially in clinical wound healing and tissue regeneration. |
References |
|