ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sumedh A. Dayal1, U.N.Puntambekar2 and P.B. Joshi3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This work presents the synthesis and evaluation of multi-walled carbon nanotubes (MWCNTs) reinforced silver-matrix nanocomposite materials that were prepared by a chemical route commonly known as Electroless Coating / In-Situ reduction. The silver nitrate was used as a starting material and was reduced to silver over the surface of carbon nanotubes by the in-situ reduction process using hydrazine hydrate as the reducing agent. This resulted in the formation of silver particles attached with uniformly dispersed CNTs. The composite so formed was characterized by using techniques like FTIR, TEM, and XRD. In this investigation the emphasis is placed on the functionalization of CNTs and its role in deagglomeration in order to achieve uniform mixing of CNTs with the silver matrix. The effect of volume per cent of carbon nanotubes on properties like relative density, Vickers hardness and electrical conductivity of the Ag-CNT nanocomposite was investigated. The results showed that the addition of carbon nanotubes up to 9 % by volume to silver matrix results in an increase in the density and Vickers hardness whereas the electrical conductivity of the composite decreases with increasing volume fraction of CNT. Beyond 9 vol. % of CNT in Ag-CNT nanocomposite a sharp drop in the electrical conductivity is observed.
Keywords |
| Nanocomposites, carbon nanotubes, functionalization, Ag-CNT electrical contacts. |
INTRODUCTION |
| Carbon nanotubes (CNTs) have emerged as a novel material in recent past because of their unique properties such as being 10 to 100 times stronger than steel, high strength to weight ratio, elastic moduli as high as 1 TPa and electrical current carrying capacity 1000 times that of pure copper, and high thermal conductivity, etc [1-3]. Such an exceptional combination of properties has made carbon nanotubes a potential candidate as a reinforcing material to develop metal matrix nanocomposites such as Ag-CNT and Cu-CNT. One of the most important future applications of CNT-reinforced metal-matrix nanocomposites is in the area of switchgear technology as make-and-break type electrical contacts [4].These unique properties of CNT make them an ideal material as second phase in silver and copper base electrical contacts used for make-and-break application owing to its ability to improve the resistance to arc erosion, resistance to contact welding and low contact resistance [5]. |
| Powder metallurgy is an established process for production of metal matrix composite materials. The important steps of this process include uniform mixing of reinforcing phase with the metal matrix in the powder form followed by its conversion to bulk solid by compaction and sintering [6]. However, factors like tendency of CNTs to agglomeration, their poor wettability by metal matrix and hence weak interfacial bonding between the metal matrix and carbon nanotubes, lead to the bulk solids having low density, higher amount of porosity as well as segregation of CNTs in the matrix. This has prompted researchers to develop alternative processes / methods to make CNT- reinforced metal-matrix nanocomposite materials [7-9]. Some of these alternative manufacturing processes are internal oxidation [10], plasma spraying [11], electrochemical deposition process [12], electroless coating [13,14], etc. Of all these processes, the electroless coating or in-situ reduction process has drawn a special attention in view of simplicity of the process, need for inexpensive equipment, improved dispersion of the CNTs in the matrix and better control over the powder particle morphology. |
| However, the major issue with the use of electroless coating process to produce Ag-CNT nanocomposite powder is that of poor adhesion and bonding between the CNT and the metallic phase because of chemical inertness of the CNT. One of the most promising routes to overcome this problem is to functionalize CNT [15]. Once the functional groups are attached to the carbon nanotubes, the electrostatic repulsive forces between the nanotubes overcome the Van der Waal’s forces of attraction between them leading to formation of a stable suspension within the solution. Functionalization thus extends their properties and in turn their application potential. Various methods developed to functionalize carbon nanotubes using covalent or noncovalent modification approach include methods like polymer wrapping, biomolecule binding, metal ion binding, solid phase mechanochemical reaction and chemical method [16-18]. The noncovalent method of functionalization using oxidative chemical process, which is quite often employed, makes use of mixture of sulphuric and nitric acid in order to generate defects on the side walls and tips of the nanotubes that can serve as anchor groups for functionalization and provide sites for chemical bonding [19]. |
| The present work deals with the development of multi-walled CNT reinforced silver-matrix nanocomposite by electroless coating / in- situ reduction method for application as the electrical contact material. The effect of volume fraction of CNTs on the relative density, the hardness and the electrical conductivity of the composite was investigated. |
EXPERIMENTAL WORK |
| The multiwalled carbon nanotubes used in this work were procured from M/s J.K. Impex, Mumbai. Figure 1 shows the TEM picture of the as-received unfunctionalized multi-walled carbon nanotubes used in this investigation displaying the greater tendency to tangling and clustering of nanotubes. The nanotubes were 11- 15 nm in diameter and 15- 20 microns in length and of 95 % purity. The silver nitrate and hydrazine hydrate used were of E-MERCK make and of AR grade. |
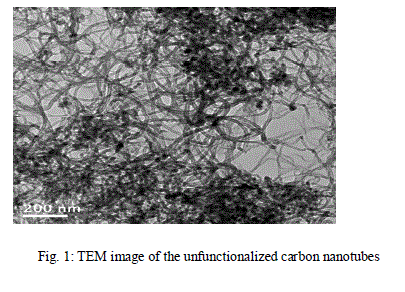 |
| Figure 2 gives the flow sheet of the processing steps followed. It is well known that for adequate interfacial bonding across the CNT/silver matrix interface, the surface roughness of CNTs must be high. Besides this the CNTs are generally having poor chemical reactivity which is attributed to its electronic structure. In view of this, the carbon nanotubes were subjected to oxidation treatment (popularly known as functionalization or surface modification treatment) in an aqueous solution consisting of sulfuric acid and nitric acid mixed in the proportion of 3:1 by volume. Surface modification/functionalization is the act of modifying the surface of a material by bringing physical, chemical or biological characteristics different from the ones originally found on the surface of a material. The process of functionalization introduces new carboxylic and hydroxyl groups onto the surface of multiwall CNTs, which helps in greater interaction between the multiwall CNTs and the metal matrix. The surface morphology of the multiwalled CNTs before and after the treatment was examined and analyzed using techniques like and Fourier Transform Infrared Spectroscopy (FTIR) and Transmission Electron Microscopy (TEM). Figure 3 gives the FTIR spectra for carbon nanotubes before and after the functionalization treatment obtained using Perkin Elmer Spectrum BX FTIR System whereas Fig.4 shows corresponding TEM images obtained with TECHNAI 20, PHILIPS transmission electron microscope. In-situ deposition or coating of metallic silver particles over the activated / functionalized CNT surfaces leads to improved adhesion between CNTs and silver matrix. |
| Hence, first of all the Ag-CNT nanocomposite powder was prepared by electroless coating / in-situ reduction process. Thereafter, the resultant powder was converted into bulk solid compacts by classical powder metallurgy process of press-sinter-repress route. |
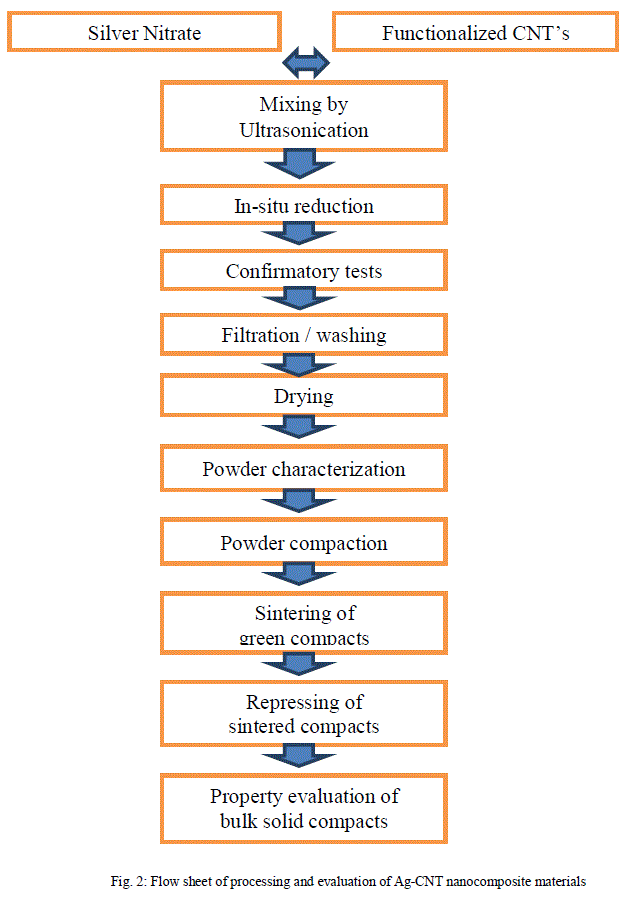 |
| As represented in the flow sheet, an aqueous solution of silver nitrate was formed by dissolving the stoichiometric amount of AgNO3 in distilled water. The weighed quantity of carbon nanotubes as per stoichiometry were added to it and thoroughly mixed by ultrasonication. The hydrazine hydrate was added as reducing agent into the aqueous solution of silver nitrate having carbon nanotubes as suspension by using a spray bulb. The ultrasonication was continued during the entire process of in-situ reduction in order to ensure uniform distribution of CNTs and silver. This resulted in in-situ reduction of silver nitrate to silver over the carbon nanotube surfaces forming Ag-CNT nanocomposite. The solution was filtered, washed and dried in an oven at 120 0C so as to get Ag-CNT nanocomposite powder. The dried powder was subjected to uniaxial die compaction on a 100 Ton capacity hydraulic press in a steel die at 300 MPa pressure and the green compacts so formed were vacuum sintered at 7000Cina resistance heating type tube furnaceat a heating rate of 7- 8 0C per minute. The sintered compacts were repressed at 600 MPa pressure for enhancement of their density. |
| The density of composite compacts was measured at various stages during the course of processing and the relative density was obtained by comparing the measured densities with the theoretical densities. The hardness of the compacts was measured using a Vickers hardness tester with a contact load of 50g. At least three hardness measurements on each sample surface at different locations were taken and the average value of these measurements has been reported. The electrical conductivity of the composite compacts was measured using a electrical conductivity meter of M/s Technofour Ltd., Pune, India make based on eddy current principle. |
RESULTS AND DISCUSSION |
| In order to examine the extent of the modification of the carbon nanotube surfaces as a result of attachment of active functional groups by mixed acid treatment, infrared spectroscopy on untreated and treated powder samples was done. Figure 3(a) and (b) show the peaks corresponding to the untreated and treated CNTs. The peak at 1115.25 in Fig. 3(b) indicates the presence of C=O bonds after modification. Whereas the peak at 1650.84 indicates the presence of C=C bonding which has shortened in height compared to the one before modification. The peak at 2928.81 indicates the presence of COOH bond which has become short and sharp. The peak at 3416.94 indicated the presence of OH group which compared to unmodified has become short and a new peak at 3742.37 indicates the presence of OH group which was not found in unmodified CNTs. |
| The comparison of FTIR spectra given in Fig. 3(a) and (b) reveals that after modification of the CNT surface by acid treatment the number of hydroxyl and carboxyl ions on the CNTs increase significantly. Thus the difference in FTIR spectra clearly indicates that the mixed acid treatment introduces new functional groups which improve the adherence of silver particles over CNT surface during in-situ reduction. |
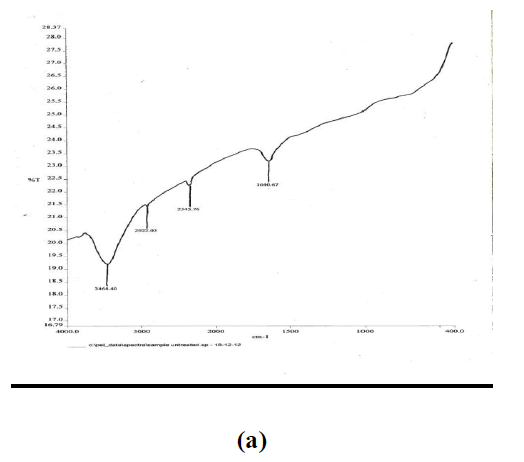 |
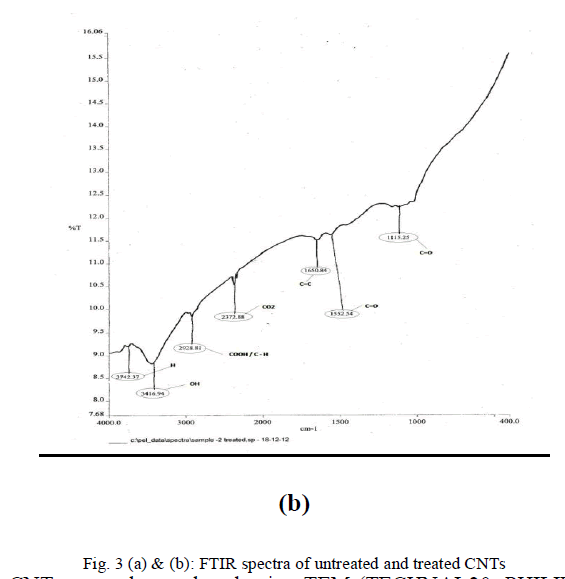 |
| The treated and untreated CNTs were also analyzed using TEM (TECHNAI 20, PHILIPS). It can be seen from the TEM picture given in Fig. 4 (a) that in untreated sample there is a high degree of clustering among the nanotubes with the diameter of the clusters around 10 - 15 nm. Figure 4 (b) shows the TEM image of MWCNTs after H2SO4 / HNO3 modification. We can see that the length of MWCNTs has become shorter after functionalization and the tubes are more isolated rather than being in the form of clusters. This is mainly because the free oxygen atoms decomposed and released by the concentrated acid that lead to electrostatic repulsion between the neighbouring nanotubes. The resultant benefit of functionalization giving the isolated and well separated carbon nanotubes is in terms of the more uniform dispersion of carbon nanotubes within the silver matrix in the Ag-CNT nanocomposite powder as well as the bulk solid compact after sintering. |
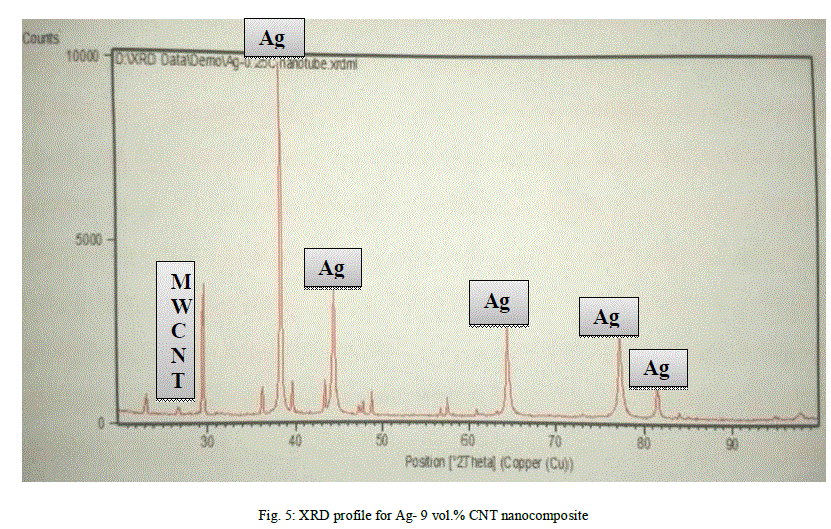 |
 |
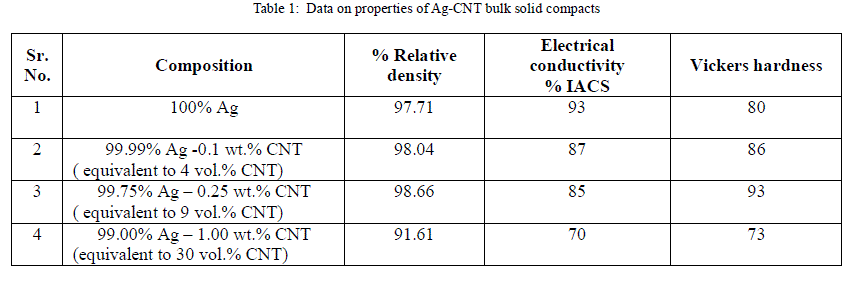 |
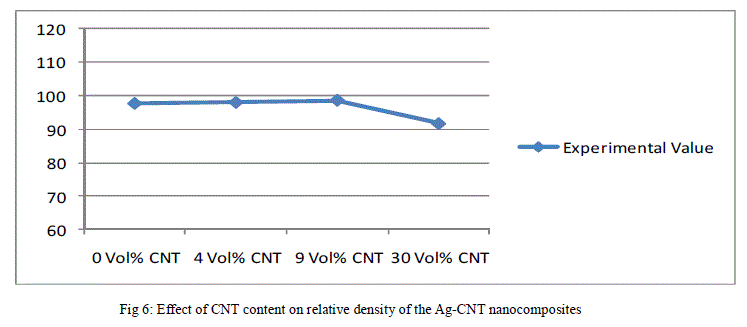
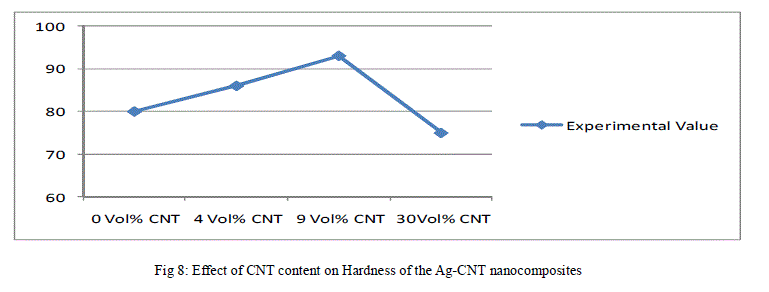
| The above data has been reported in the form plots in Fig. 6 to Fig. 8, for ease of interpretation and clear understanding. |
| Figure 6 shows the effect of volume fraction of CNTs on the relative density of the Ag-CNT nanocomposite bulk solids. Accordingly, as the volume per cent of CNT in Ag-CNT nanocomposite increases, the relative density increases up to certain point and then it starts decreasing. As shown in the plot given in Fig. 6, there is a gradual increase in relative density up to 9 volume % of CNT in Ag-CNT nanocomposite and thereafter the density decreases, appreciably. The decreases in density at higher volume % of CNT may be attributed to the formation of clusters of nanotubes that impede the diffusion of silver into small spaces between the nanotubes, resulting in a non-uniform distribution of two materials within the composite and also the formation of pores during sintering. Besides this, the CNTs have high elastic modulus and hence they spring back partly during sintering leading to the expansion of the composite and a decrease in relative density. |
| The relative density of Ag-CNT nanocomposite bulk solid compacts after repressing is higher than that of assintered compacts because of plastic deformation of silver and hence repressing is employed to increase the relative density of the composite. |
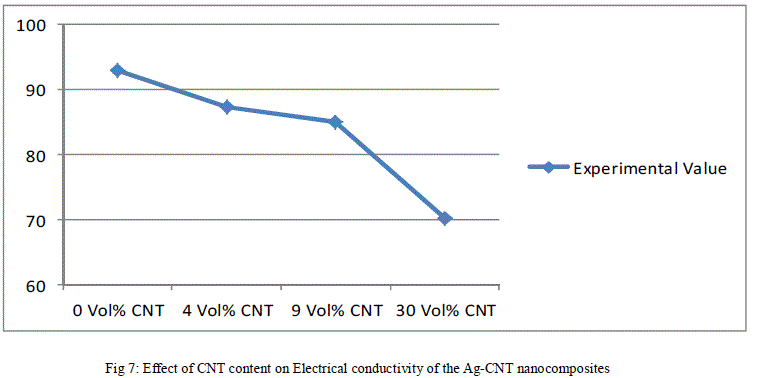 |
| The effect of volume fraction of CNT on electrical conductivity of Ag-CNT nanocomposite bulk solids has been shown in Fig. 7. As the volume content of CNT increases the electrical conductivity of Ag-CNT nanocomposite material decreases. At relatively lower volume fraction of CNT i.e. up to about 9 vol.%, the electrical conductivity decreases slowly but thereafter there is a fast reduction in electrical conductivity. Although the CNTs possess high electrical conductivity, with increase in CNT content beyond a certain optimum level, the high surface area of the nanotubes creates a large interfacial area between the nanotubes and silver, resulting in increased scattering of electrons during electrical conduction leading to decrease in electrical conductivity. Not only this, as the amount of nanotubes within the composite increases more defects are generated due to cluster formation. This combined with lattice strain in silver matrix around the nanotubes acts as a barrier to electron motion. The resultant effect of all this is reduced electrical conductivity at high levels of CNT. |
 |
CONCLUSION |
| It can thus be concluded that it is possible to produce silver - CNT nanocomposite materials by using novel powder synthesis technique of electroless coating based on nanotechnology principle as an alternative to existing conventional practice of blending/mixing of silver and CNT constituent powder. However, surface modification of the carbon nanotubes is a mandatory step before electroless silver coating. This is in view of the low chemical reactivity of CNTs and high interfacial energy difference between the carbon nanotubes and the silver matrix. Using electroless coated Ag- CNT nanocomposite powders; it has been possible to attain the density level of 98.66 % of theoretical density, the electrical conductivity of the order of about 85 % I.A.C.S. and microhardness equal to 93 Hv for Ag-9 vol % CNT nanocomposite bulk solids. |
ACKNOWLEDGEMENTS |
| We are thankful to thank Dr. V. Shrinet for supporting this research work. Special thanks to Dr. G.S. Grewal for giving the expert guidance at various stages during the course of this investigation. The help rendered by Dr. BharatiRehani, Dr. KantiBhambhaniya, Dr. Hemang Patel and Mr. NitinBatra is also gratefully acknowledged. |
References |
|