ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Seenivasagan, R1., Rajakumar, S2. and Ayyasamy P.M3.
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The efficient nitrate reducing bacterial species was isolated from water sample collected from Salem district of Tamil Nadu, India. The bacterial strain Bacillus sp. (SW-59) selected as potential organism and it showed a better nitrate removal efficiency in the nitrate reduction test. Influence of various carbon sources, incubation temperature and pH on nitrate removal was studies in mineral salts medium (MSM) supplemented with 100 mg/L of nitrate. Efficient nitrate reduction of 84.02% was attained in MSM amended with 0.5% of starch at an optimum temperature of 35°C and pH 7. This observation has led to the conclusion that the bacterial strain (SW- 59) showed better efficiency for nitrate removal from synthetic nitrate-rich wastewater
Keywords |
| Nitrate removal, Bacillus sp., Carbon source, Starch, Temperature, pH, |
I. INTRODUCTION |
| Water wealth was under threat due to one of the most widely existing elements in nature, nitrogen which is an essential element for creatures growing and breeding [1]. Increasing this nitrate concentrations constitute a serious environmental problem, especially groundwater is a source of drinking water [2]. Agriculture is the most extensive source of nitrate to groundwater and additional sources include landfill leachate, leaking septic tanks, animal waste disposal, treated wastewater discharged to rivers and municipal storm water runoff [3, 4]. Nitrate due to its high water solubility imposing a serious threat and causing ecological disturbances [5]. The ecological effects contributing to eutrophication of rivers, hypoxia, toxic algal blooms, shifts in the food chain, loss of biodiversity, loss of fish stocks, habitat degradation in streams, deterioration of water sources, lakes and elevated N2O emissions [6]. Nitrate is transformed to nitrite in the digestive system which creates the condition known as methemoglobinemia also called as Blue Baby Syndrome [7]. |
| Due to several hazards by nitrate, proper handling is needed to solve the nitrate contamination in ground water and soil. The physical and chemical technologies for the nitrate removal are relatively expensive and produce concentrated waste brines requiring further treatment or disposal [8]. The biological denitrification is an alternative technology and there are no secondary waste products [9]. Microbes have been applied to clean up groundwater, soils, lagoons, sludge and process-waste streams [10]. Main parameters influencing denitrification are oxygen concentration, the availability of C and NO3 -, temperature and pH [11]. Hence, the present study was aimed to investigate the removal of nitrate by Bacillus sp. (SW-59) in the synthetic medium amended with various carbon sources. Effect of various temperatures and pH on the nitrate removal was also studied to find out suitable optimization. |
II. MATERIALS AND METHODS |
| A. Bacterial isolation |
| The bacterial strains were isolated from the water samples collected from Salem district of Tamil Nadu, India. The bacterial strains were screened by nitrate reduction test using potassium nitrate broth for the removal of nitrate. The ability of the isolate to reduce nitrate to nitrite and ammonium were determined by the addition of 1 mL Nessler’s reagent to the enriched broth. Based on the deep intensity of the color, the isolate Bacillus sp. (SW-59) was selected as best nitrate reducer. |
| B. Optimization of carbon substrates on nitrate removal |
| Mineral salt medium (MSM) with 100 mg/L of NO3 − and 1% of different carbon substrates such as glucose, starch and cellulose were prepared and sterilized. About 104 CFU/mL of SW-59 was inoculated to the medium and kept in a shaker (120 rpm) at room temperature for 84h. The samples were drawn aseptically at regular intervals of 12, 24, 36, 48, 60, 72 and 84h and the bacterial growth was analyzed using UV-Vis spectrophotometer (Cyberlab UV100, USA). The reduction of nitrate (phenol disulphonic acid method) and formation of nitrite (NEDA method) and ammonium (Nessler’s reagent) in the medium were estimated by standard methods [12]. |
| C. Effect of various concentration of starch on nitrate removal |
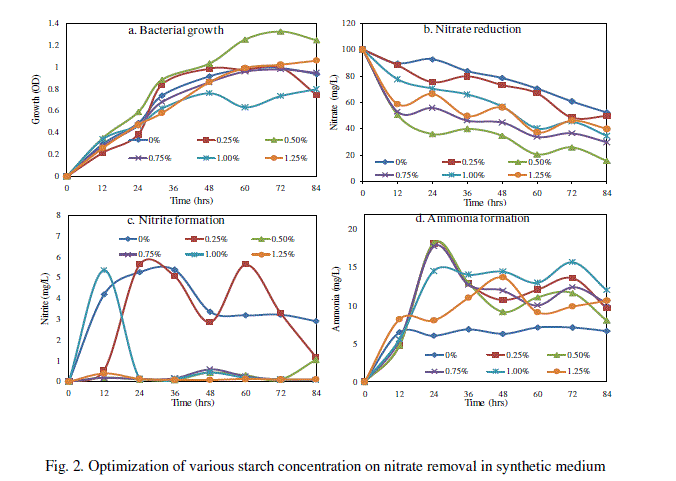
| Starch was found to be ideal carbon source in nitrate removal when compared to other carbon sources. Hence, MSM was prepared with 100 mg/L of NO3 − and 0.25, 0.50, 0.75, 1.0 and 1.25 % concentrations of soluble starch and sterilized. To this one ml of inoculum containing 104 CFU/mL of SW-59 was inoculated and kept in a shaker (120 rpm) at 35oC for 84h. The samples were aseptically drawn at regular intervals and the bacterial growth, nitrate, nitrite and ammonium were estimated. In this study, starch at 0.5% exhibited a significant nitrate removal. Hence, starch at 0.5% was optimized as a potential concentration for further nitrate removal studies. |
| D. Optimization of various temperatures on nitrate removal |
| MSM with 100 mg/L of NO3 − and supplemented with 0.5% starch was prepared and sterilized. Cells of SW-59 (104 CFU/ml) were inoculated to the previously prepared medium and kept in a shaker (120 rpm) at various temperatures 25, 30, 35, 40 and 45oC for 84 h. At every 12h, the bacterial growth, nitrate, nitrite and ammonium contents in the medium were determined. |
| E. Optimization of various pH on nitrate removal |
| MSM with 100 mg/L of NO3 − at various pH (5, 6, 7, 8 and 9) and supplemented with 0.5% starch was prepared and sterilized. About 104 CFU/ml of SW-59 was inoculated to the medium and kept in a shaker (120 rpm) at presumptively optimized temperature of 35°C for 84 h. Every 12 h, the bacterial growth, nitrate, nitrite and ammonium were estimated. |
| F. Analysis of Linear correlation (R2 score) |
| Effect of different carbon sources, starch concentrations, temperatures and pH on nitrate reduction was carried out and the results were interpreted through correlation (R2 score) using Microsoft Excel (2007 version) to confirm their optimum conditions [13]. |
III. RESULTS AND DISCUSSION |
| The bacterial species Bacillus sp. (SW-59) was isolated from the water samples of the Salem district and showed better efficiency for the nitrate removal. Bacillus sp. can use NO3 − as their primary electron acceptor in aerobic condition when low oxygen availability restricts their metabolism [14]. Thus the bacteria supplies electron from organic degradation and enhances NO3 − reduction. |
A. Effect of various carbon sources on nitrate removal |
| Effect of various carbon sources on nitrate removal was carried out and the results are given in Fig. 1. Among the three carbon sources, starch exhibited the highest nitrate reduction rate which was 82.9% and maximum bacterial growth was 1.219 OD at 84 h. The formation of nitrite was found to be maximum 1.213 mg/L (Fig. 1c) in glucose at 12 h and ammonium formation 11.04 mg/L (Fig. 1d) was higher in synthetic medium amended with glucose at 24h. The addition of organic source and carbon energy is required for denitrifications process because in the process of biological denitrification, nitrates are used by bacteria as a final electrons acceptor in the electron transport chain and are reduced to nitrogen [15]. |
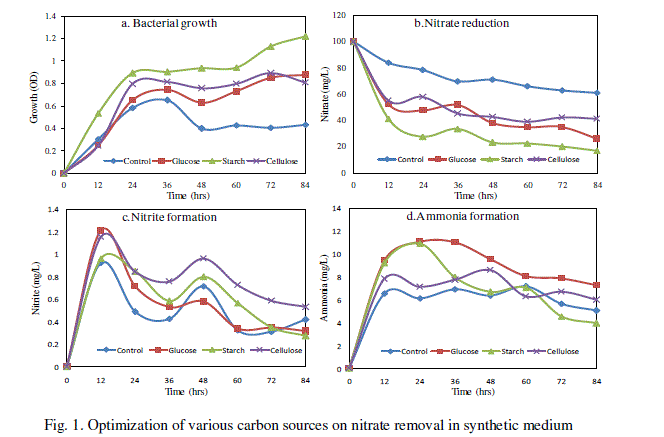 |
| B. Effect of various starch concentrations on nitrate removal |
| The Bacillus sp. (SW-59) reduced maximum level of nitrate about 84.08% and bacterial growth of 1.254 OD in synthetic medium with 0.5% starch after 84h (Fig. 2). The nitrite formation was found to be maximum 5.639 mg/L in synthetic medium supplemented with 0.25% starch at 24 h and then decreased thereafter (Fig. 2c). The level of ammonium 18.24 mg/L was also formed in synthetic medium with 0.5% starch at 24h (Fig. 2d). Hence, starch at 0.5% concentration was used as selective carbon source for further experiments of removal of nitrate. Our results are correlated with Kim et al. [16] where the use of starch as a carbon source in the on-site biological treatment of nitrate in ground water was successful with a nitrogen removal efficiency of 99.5% and corresponding to 4.3 g of soluble starch. |
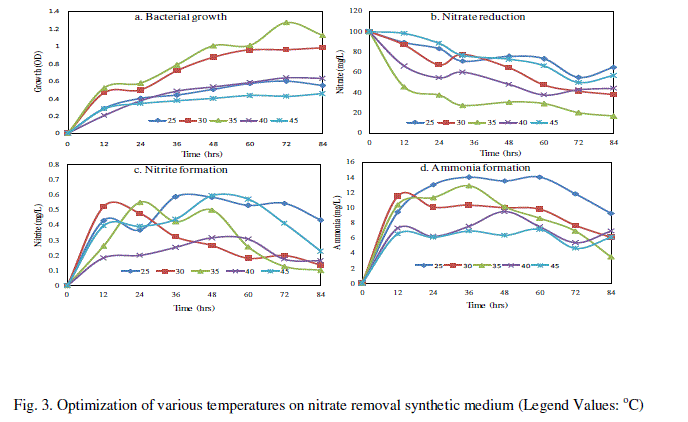
| C. Effect of various temperatures for nitrate removal |
| The bacterial isolate SW-59 reduced maximum level of nitrate 83.23% at 35°C for 84 h compare than other temperature (25, 30, 40 and 45°C) and bacterial growth in 1.271 OD at 72h (Fig. 3). The nitrite formation of about 0.596 mg/L in 45oC at 48h and ammonium showed 14.04 mg/L at 25oC (Fig. 3c and d). There were several studies that had investigated the effect of temperature on denitrifications rates. Muller et al. [17] studied the denitrification rates found were certainly upper limits because the conditions in vitro supply of NO3 − and control of temperature, an aerobiosis and moisture-favor denitrifications. |
 |
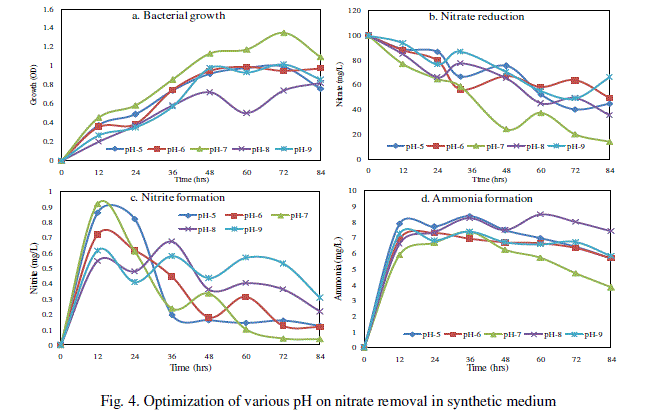
| D. Effect of pH for nitrate removal |
| The maximum of nitrate was reduced to 85.88% and bacterial growth of about 1.348 OD in synthetic medium amended with 0.5% starch in pH 7 at 35°C for 84hr when compare than other pH 5, 6, 8 and 9 (Fig. 4). The maximum nitrite 0.917 mg/L at pH 7 for 12h and ammonium 8.48 mg/L at pH 8 for 60h (Fig. 4c and d). However, studies on denitrification in pH were scarce. Saleh-Lakha et al. [18] stated that temperature and pH plays a role in influencing denitrifier growth, metabolism, gene expression and subsequent denitrification. |
 |
| E. Analysis of linear correlation (R2 score) for nitrate reduction |
| The correlation between the different carbon sources, effects of starch concentration, temperature and pH on nitrate reduction were found to be significant level of 0.959 (Fig. 5). The removal rates of nitrate were plotted versus different carbon source, starch dosage, temperature and pH; the data were plotted on a nearly straight line, indicating that the reaction was pseudo-first-order with respect to optimized conditions. Our result was similar to Song et al. [19] in which good agreement with the pseudo first and second order kinetic models with the relatively high correlation coefficients (R2 > 0.98). |
 |
IV. CONCLUSION |
| Bacillus sp. SW-59 was isolated from water samples collected from Salem district, Tamil Nadu and had better efficiency in terms of nitrate reduction. The nitrate reduction by SW-59 was severally influenced by various carbon sources, temperature and pH. Starch at 0.5% could be used as the best carbon source, the temperature at 35°C and neutral pH were optimum in the synthetic wastewater. The results of R2 score showed a good linear correlation between the parameters. Thus, the Bacillus sp. (SW-59) could be reduced nitrate below the permissible limit within 24 h. |
V. ACKNOWLEDGEMENT |
| The Authors are thankful to University Grants commission (UGC), New Delhi for providing financial support to carryout this research work. |
References |
|