ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K.B.Girisha1, Dr.H.C.chittappa2,
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
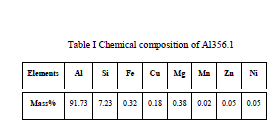
In this research paper Aluminum alloy (Al356.1) matrix composite reinforced with Zirconium Oxide nano particle in the weight % of 0.5, 1, 1.5, and 2.0 %. Reinforcement process is carried out by stir casting method Microstructure and mechanical properties of Nano composites. The nanocomposites were characterized by scanning electron microscope (SEM) and X-ray diffraction (XRD). Wear test were carried out in order to identify the mechanical properties The results reveal that the composites containing 1.5 wt % reinforcement particle fabricated at 850oC have homogenous reinforcements of ZrO2 in Al356.1 wear properties and hardness strength also improved
Keywords |
| Metal matrix composites, Nano structured materials, ZrO2/Al356.1 composites |
INTRODUCTION |
| Nowadays, demands for developing metal matrix composites for use in high performance applications, have been significantly increased [1]. Among these composites, aluminium alloy matrix composites attract much attention due to their lightness, high thermal conductivity, moderate casting temperature, etc. [2,3]. Various kinds of ceramic materials, e.g. SiC, Al2O3, MgO and B4C, are extensively used to reinforce aluminium alloy matrices. Superior properties of these materials such as refractoriness, high hardness, high compressive strength, wear resistance, etc. makes them suitable for use as reinforcement in matrix of composites [4–11]. Nevertheless, low wet ability with molten metal’s and density differences increases their tendency toward agglomeration, which deteriorates mechanical properties. Numerous attempts have been made to overcome the mentioned weakness [12–14]. As aerospace technology continues to advance, there is a rapidly increasing demand for advanced materials with high mechanical and thermal capabilities for such ultra high applications [15]. Its application also stretched to automobile, electronic and computer industries to replace the existing materials including plastics [16]. |
| The early 1990s are considered to be the renaissance for Al as structural material due to environmental concerns, increasing safety and comfort levels. A significant improvement in the properties of Al alloys, reduced fuel consumption because of Lightweight has made huge demand from automobile industry [17, 18]. This growing requirements of materials with high specific mechanical properties with weight savings has fuelled significant research activities in recent times targeted primarily for further development of Al based composites [19–21]. A recent industrial review revealed that there are hundreds of components from structural to engine in which aluminium alloy is being developed for variety of applications [22]. It is also predicted that for Al alloys demand increased globally at an average rate of 20% every year [23]. It is noticed that the limited mechanical properties (strength and hardness) of Al and its alloys adversely affect its applications in automobile and aerospace industries [24, 25]. This remains one of the major concerns in its fabrication to suit its application in recent days. |
EXPERIMENTAL PROCEDURE |
| A. Synthesis of ZrO2 material: |
| The ZrO2 Nano powder was prepared by dissolving zirconyle nitrate (ZrO (NO3)2) and Crystal sugar (C6H12O6) as fuel in a minimum quantity of double distilled water is taken in a ceramic crucible. The crucible containing the solution was introduced into a preheated muffle furnace maintained at 850 ± 5º C. The solution initially boils and undergoes dehydration followed by decomposition with the evolution of large amount of gases. The solution boiled resulting in a transparent gel. The gel then formed white foam, which expanded to fill the vessel. Shortly thereafter, the reaction was initiated somewhere in the interior and a flame appeared on the surface of the foam and proceeded rapidly throughout the entire volume, leaving a white powder with an extremely porous structure. The entire combustion process for producing ZrO2 powder takes place. The reaction for combustion synthesis in the present case can be written in equation (1) |
 |
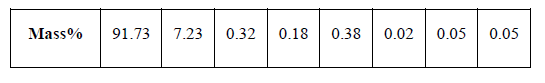 |
| The samples were prepared using a resistance furnace, equipped with a stirring system. After smelting of aluminum ingots, ZrO2 Nano powder was added to the molten metal and stirring was carried out at constant rate of 150rpm for 20min. according to the results of literature and previous works [19, 20]. Nano-powder ZrO2 (0.5, 1.0, 1.5, and 2.0wt %) added to the molten metal during stirring. The casting was performed at 8500C. Steel circular die was used for casting of specimens. Finally, specimens fabricated in five various conditions were prepared for subsequent Micro structural and mechanical analyses. |
RESULTS AND DISCUSSION |
| A. X-ray Diffractometer (XRD) Studies |
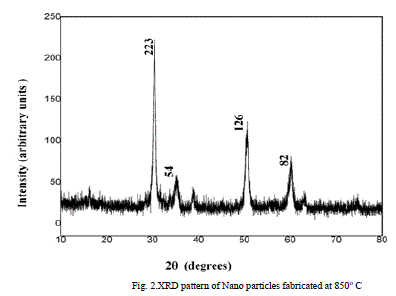
| The powder X-ray diffraction studies were carried out using Phillips X-raydiffractometer (model PW 3710) with Cu Kλ radiation(λ = 1.5405 AÃâ¹ÃÅ¡ ) The X-ray diffraction pattern of nano-ZrO2 powder confirms the crystalline phase and mean crystal size determined was around 40 nm. In the XRD observations three Strongest peaks shown in Fig. 2 were detected with Miller indices (223), (054), (126), and (082) corresponding to Bragg angles 30°, 36°, 51°and 59°respectively. The characteristic peaks are higher in intensity which indicates that the products are of good crystalline nature. No peaks corresponding to impurities are detected, showing that the final product is purely ZrO2 Nano powder. It is observed that intensity of the peaks increases with thermal treatment due to Agglomeration, which means that the crystalline has been improved. The full width at half maxima of major peaks decrease and confirms the grain size growth. |
 |
 |
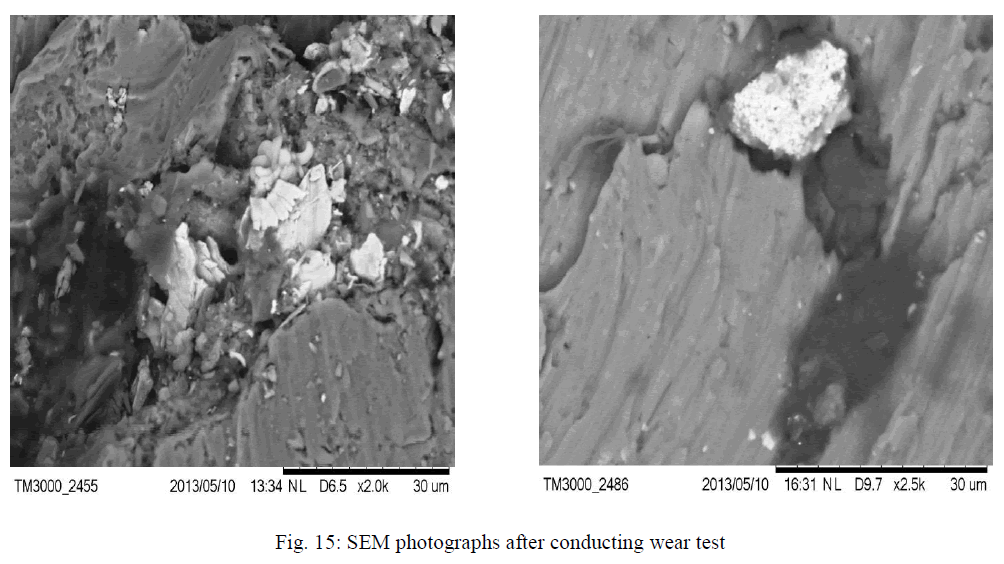
| The Nano composites were found to be agglomerated when analyzed by scanning electron microscopy (SEM: JEOL, Japan, JSM 840A) studies shown in Fig 4, 5, 6, 7, 8, It can be observed that the ZrO2 crystallites have no uniform shape. This believed to be related to the non-uniform distribution of temperature and mass flow in the combustion flame, due to the high surface energy of the particles and from the SEM there is such difference was observed for different wt % of ZrO2 dispersed aluminum powder. In micrographs also observing that the big particles are aluminum powder and the very small particles surrounded by that are ZrO2. |
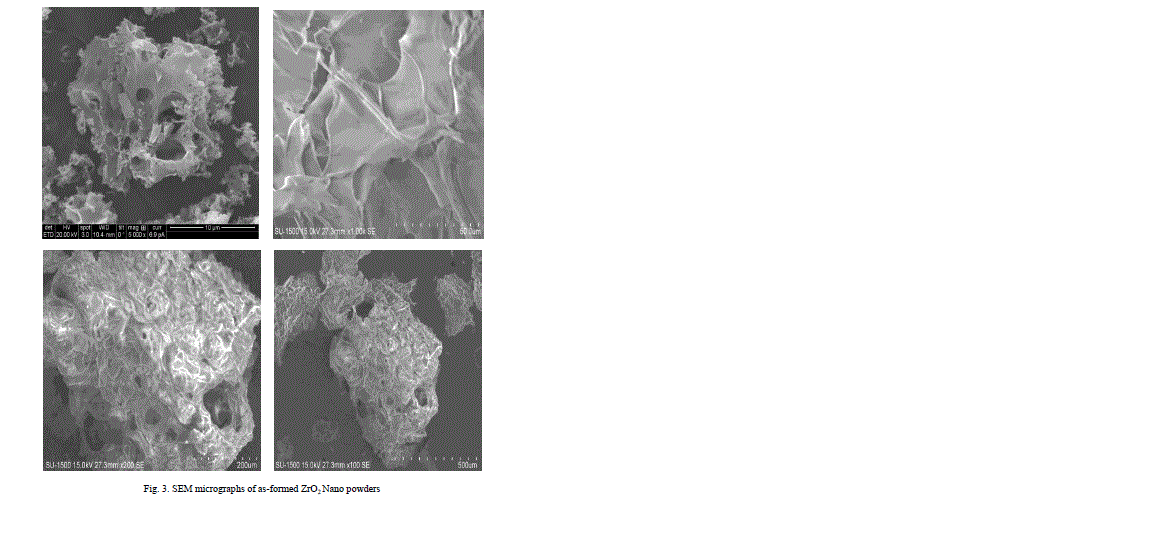 |
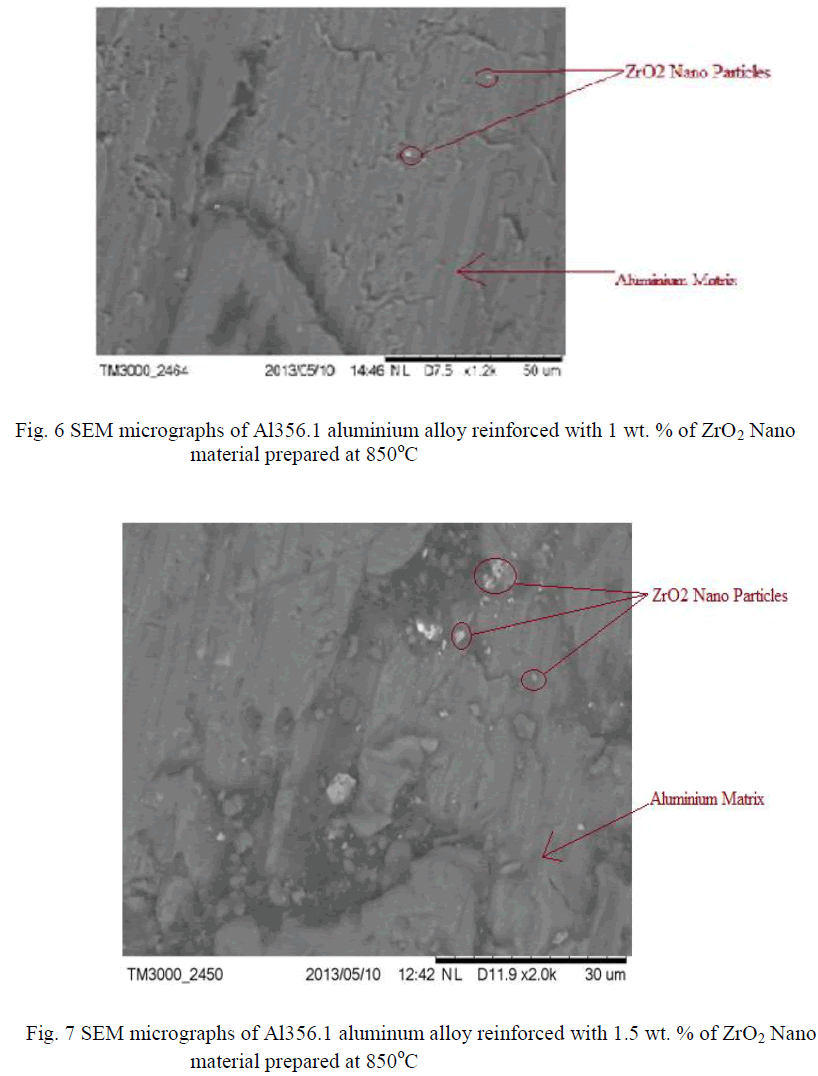 |
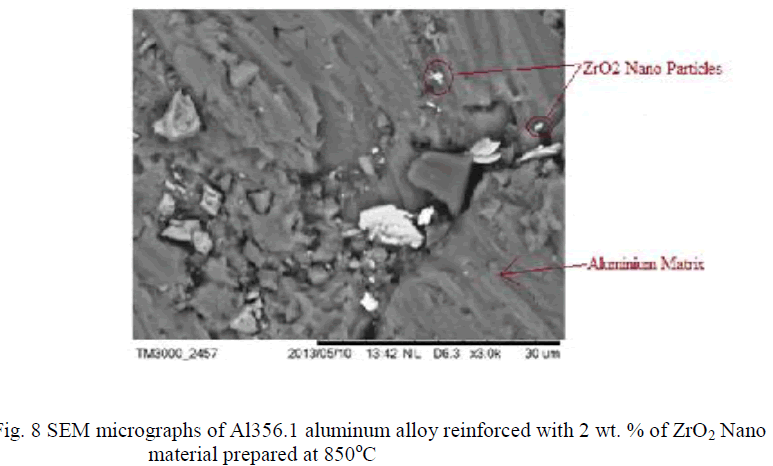 |
| C. Wear properties: |
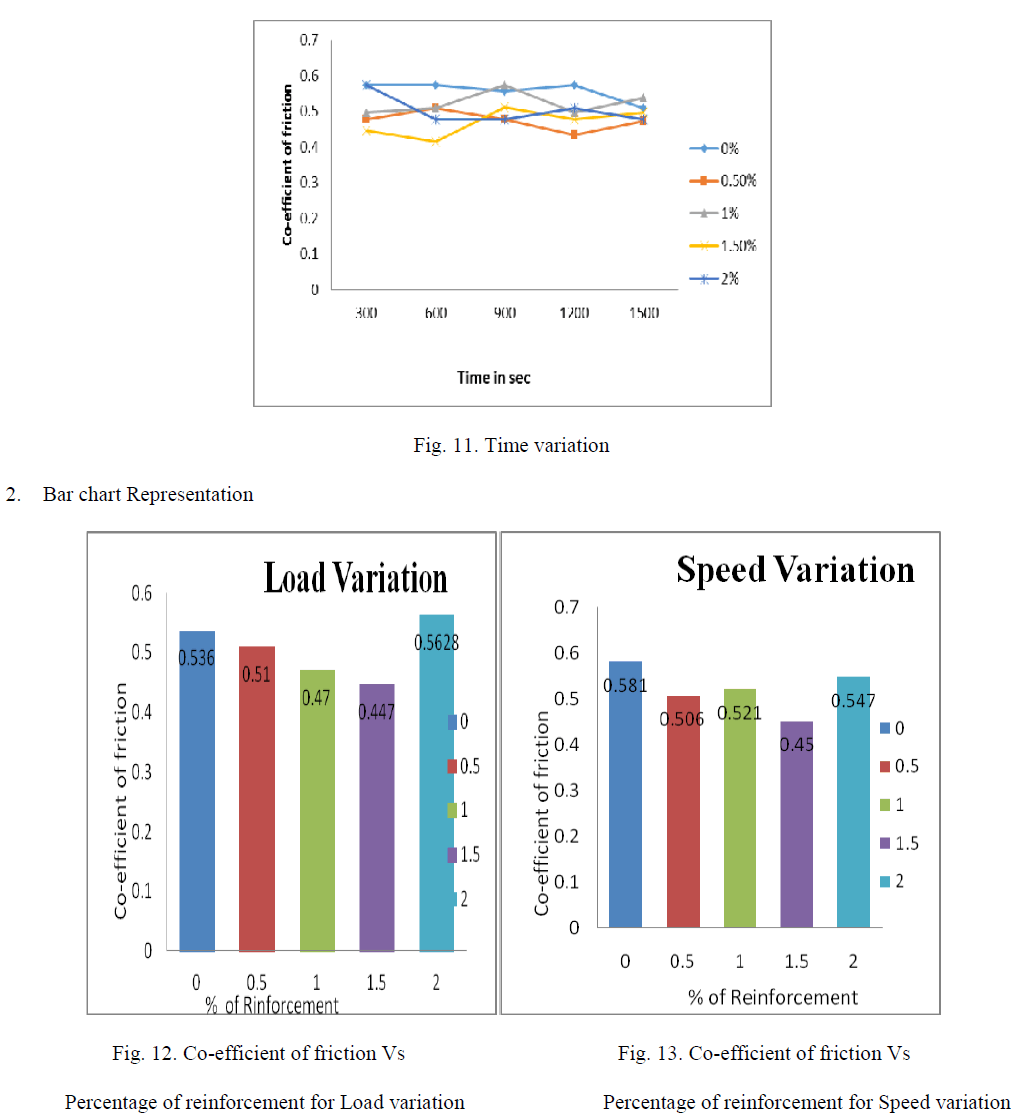
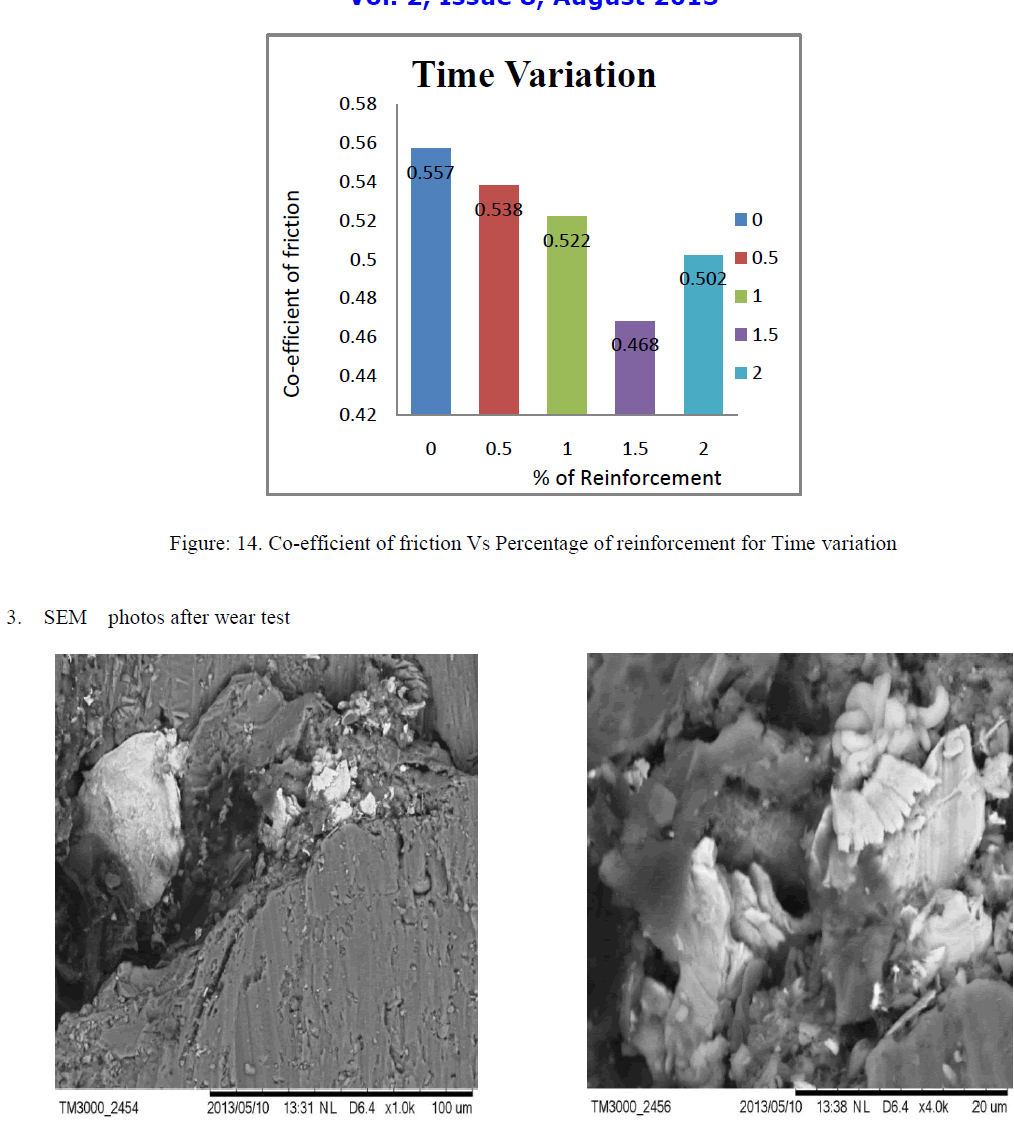
| As the wt % of ZrO2 reinforcement increase in Al356.1 aluminium alloy the coefficient of friction (cof) decrease up to 1.5wt % ZrO2 and again coefficient friction increases above 1.5 this can be shown by linear and bar graphs bellow, as load, speed and time increases with very small increment in cof of 1.5 wt% Nano ZrO2 composites which are shown by linear bar graphs |
| D. Percentage Wise Comparison Of Co-efficient Of Friction |
| 1. Linear Representation |
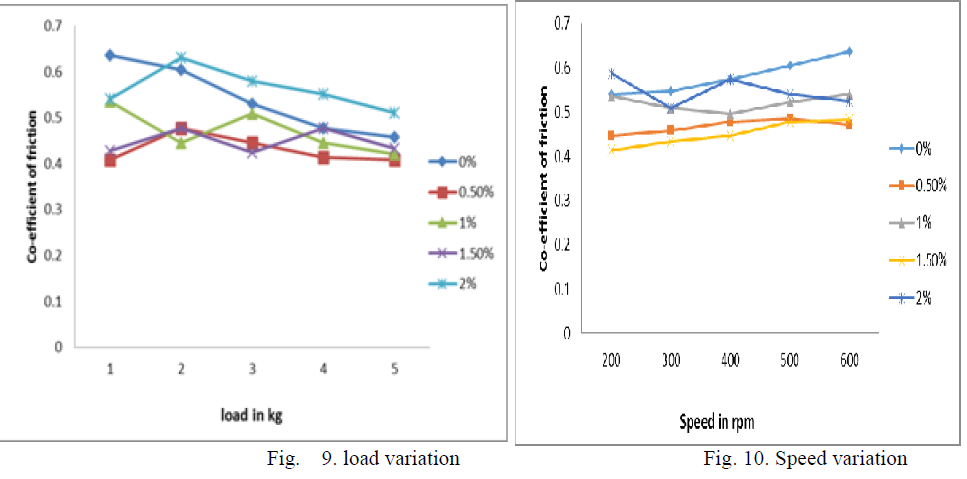 |
 |
 |
 |
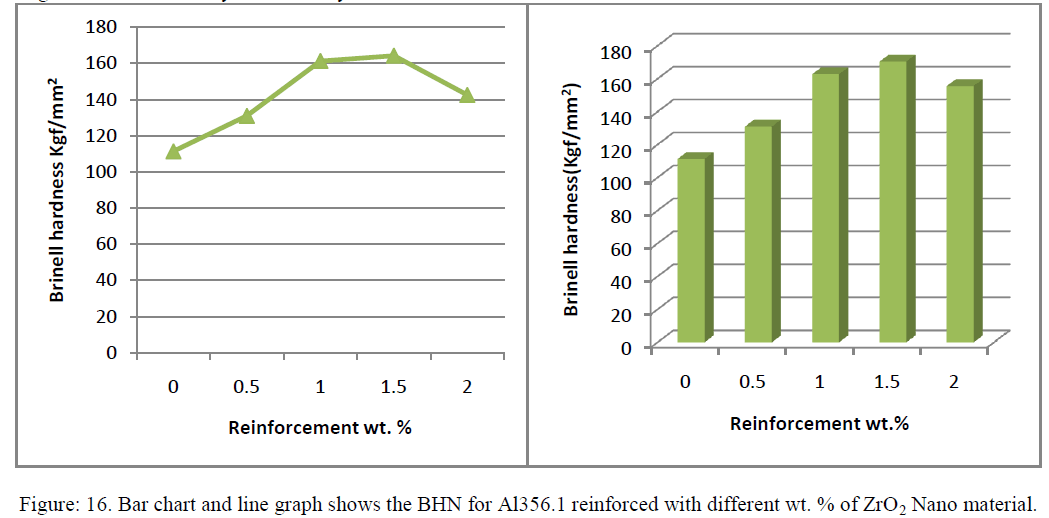
| D. Hardness test: |
| The results of micro hardness test conducted on NMMCs samples revealed an increasing trend in matrix hardness with increase reinforcement content of 1.5% (see graph .16.) Results of hardness measurements revealed that an increase in the reinforcement content leads to a significant increase in the hardness and can be attributed primarily to: the presence of harder ZrO2 Nano materials a higher constraint to the localized deformation during indentation due to their presence and reduced grain size. Results shows that as wt% of reinforcement increases the hardness value increases up to 1.5 wt % after that goes on decreases may be due to crystal structure of Nano materials |
 |
CONCLUSION |
| Al356.1 aluminum alloy reinforced with Nano sized ZrO2 was successfully fabricated via stir casting method. Reinforcement particles were well distributed in the matrix of composites. However, particle agglomeration was observed in composites with high content of ZrO2. Therefore stir casting was found as suitable method for fabrication of this kind of composites. Mechanical characterization revealed that the presence of nano-ZrO2 particulates in Al matrix significantly improved hardness and wear properties Al356.1 Aluminum alloy reinforced with (0.5%, 1.0%, 1.5%, and 2.0%) 1.5% of Zirconium Nano material. Has reviled that the wear properties of the reinforced composite is enhanced compared to the base metal. |
References |
|