ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Elsherbiny. H. El-Sayed1, Mohamed. Abd El-Moneim2, Ibrahim. M. El-Deen2, Ahmed. M. Mossalam3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
1,2-Bis[(o-hydroxy-1-naphthaldiene)amino]-ethane 3, 2-hydroxy-1-naphthaldazine 4 and thiosemicarbazone derivative 6 were prepared by ring opening of coumarin derivatives 2a,b and 10 with ethylenediamine, hydrazine hydrate and thiosemicarbazide. Acetylation of compounds 4 and 6 give 2-acetoxy-1-naphthaldazine 5 and triacetyl 7 derivatives. 5-Bromo salicylaldazine 11 was prepared via ring opening of 5-bromo-3-benzoylamino coumarin 9 with hydrazine hydrate. The mass spectral fragmentation patterns of some prepared compounds were investigated to elucidate the structure of the synthesized compounds. Biological studies of some prepared compounds were carried out to investigate their antioxidant and anticancer activities. It was found that compounds 4, 7 and 11 were active against antioxidant. Compounds 7 and 11 were also highly active against MCF-7 and HEPG-2 cell lines.
Keywords |
| Diazine derivatives, Thiosemicarbazone, Antioxidant, Antituomer. |
I. INTRODUCTION |
| Coumarins are present in natural and synthetic compounds possessing biological activity. They are acting at different stages of cancer formation. Some of them have cytostatic properties and the other have cytotoxic activity [1-3]. Two naturally occurring coumarins have been found to exhibit cytotoxicity against a panel of mammalian cancer cell lines [4]. In view of their importance as drugs, biologically active natural products and in other related applications, extensive studies have been carried out on the synthesis of coumarin compounds in recent years. In a previous work, El-Deen et al [5-10] reported the synthesis of 3-substituted coumarins (2a,b , 9 and 10) from 2- hydroxynaphthaldehyde and 5-bromosalicylaldehyde with active methylene (such as ethylacetoacetate, diethylmalonate and n-benzoylglycine). This paper describes the ring opening of 3-substituted coumarins (2a,b , 9 and 10) with nitrogen nucleophilic reagents. The electron impact (EI) ionization mass spectral fragmentation of the prepared compounds is also described. |
II. RESULTS AND DISCUSSION |
| 1. Chemistry |
| 3-Acetyl benzocoumrin 2a and 3-ethoxycarbonylbenzocoumarin 2b were prepared via cyclocondensation of 2- hydroxyl-1-naphthaldehyde with active methylene (namely, ethylacetoacetate and diethylmalonate) in presence of piperidine. Reaction of 3-substituted-benzocoumrin 2a,b with ethylenediamine and hydrazine hydrate in ethanol under reflux yielded the corresponding 1,2-bis[(o-hydroxy-1-naphthaldiene)amino]-ethane 3 and 2-hydroxyl-1- naphthaldazine 4 (Scheme1) respectively. |
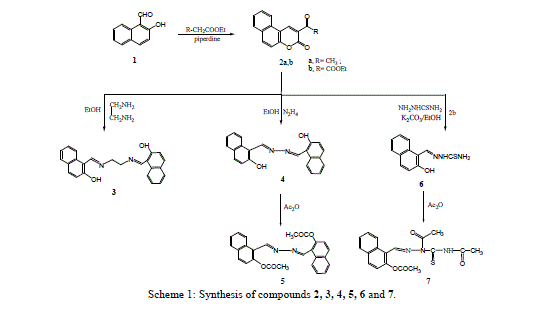 |
| The nucleophilic attack of compound 2 at 2- and 4-positions leads to the formation of compounds 3 and 4 by ethylenediamine and hydrazine hydrate resulted in fission and ring opening as shown in Scheme 2. |
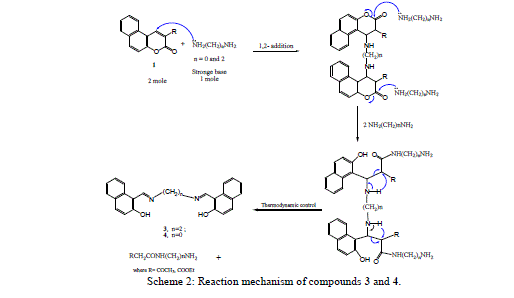 |
| Treatment of 3-ethoxycarbonyl benzocoumarin 2b with thiosemicarbazide led to the formation of 2-hydroxy-1- naphthaldehyde thiosemicarbazone 6. The 2-hydroxyl-1-naphthaldazine 4 and thiosemicarbazone 6 derivatives were transformed to 2-acetoxy-1-naphthaldazine 5 and triacetyl thiosemicarbazone 7 derivatives (Scheme1) via reflux with acetic anhydride. 5-Bromo-3-benzoylamino coumarin 9 and 3-benzoylamino benzocoumarin 10 were prepared via cyclocondensation of N-benzoylglycine 8 with aromatic aldehydes (namely, 5-bromo-2-hydroxyl benzaldehyde and 2- hydroxy-1-naphthaldehyde) in presence of fused sodium acetate and acetic anhydride under fusion. Hydrazinolyses of compounds 9 and 10 with hydrazine hydrate under reflux in ethanol to give the corresponding 5-bromosalicyladazine 11 and 2-hydroxyl-1-naphthaldazine 4 (Scheme 3) respectively. |
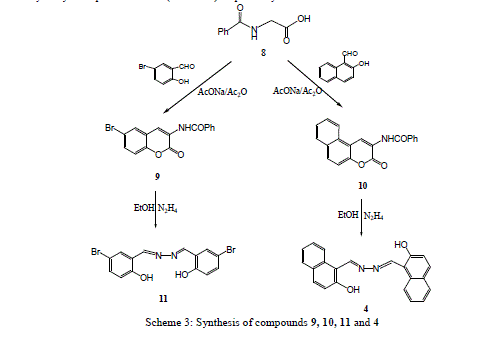 |
| 2. Mass spectroscopy |
| The mass spectral decomposition modes [11,12] of 1,2-Bis[(o-hydroxy-1-naphthaldiene)amino]ethane 3, 2-hydroxy(or 2-acetoxy)-1-naphthaldazines 4, triacetyl thiosemicarbazone derivative 5 and 5-bromosalicyladazine 11 have been suggested and investigated. |
| 1,2-Bis[(o-hydroxy-1-naphthaldiene)amino]ethane 3 |
| The molecular ion of compound 3 (m/z 368, Fig. 1), underwent fragmentation via pathway A to produce a stable ion peak at m/z 184, corresponding to 2-hydroxyl- naphthaldiene amino methylene radical cation. The loss of hydroxyl group (OH) from the stable ion with m/z 184 resulted in an ion at m/z 167. The ion of m/z 167 fragmented to give an ion at m/z 141, which lost a cyano group. The ion of m/z 141 underwent a loss of acetylene molecule to give a peak at m/z 115. |
| Furthermore, the molecular ion of compound 3 (m/z 368, Scheme 4) underwent fragmentation via pathway B by loss of 2-hydroxy-1-naphthaldiene amino to give a peak at m/z 198. Loss of the ethylene molecule from the ion at m/z 198 gave a peak at m/z 170. It is further lost a cyano, fromyl and two molecules of acetylene compound to give peaks at m/z 144, 115, 89 and 63, respectively. |
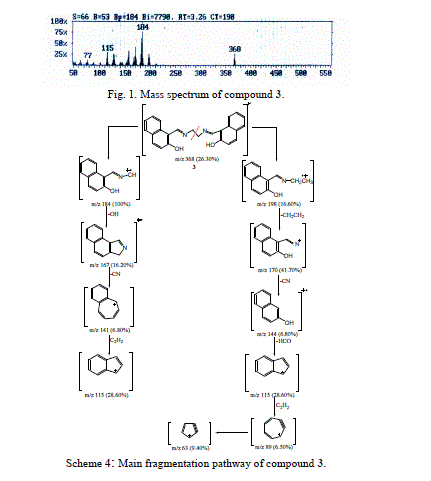 |
| 2-Hydroxy (or 2-acetoxy)-1-naphaldazine (4 and 5) |
| The mass spectra of compounds 4 and 5 showed intense molecular ion peaks at m/z 340 and 424 consistent with the molecular formula C22H16N2O2 and C26H20N2O4, respectively. The molecular ion of compound 4 (Fig. 2, m/z 340) fragmented via the pathway A and gave the stable ion of m/z 170. The stable fragment ion at m/z 170 was broken to give fragment ion at m/z 153 which lost the hydroxyl group (OH). The fragmentation led to ions of m/z 127 and 77, respectively. |
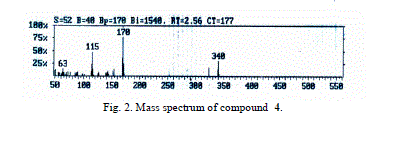 |
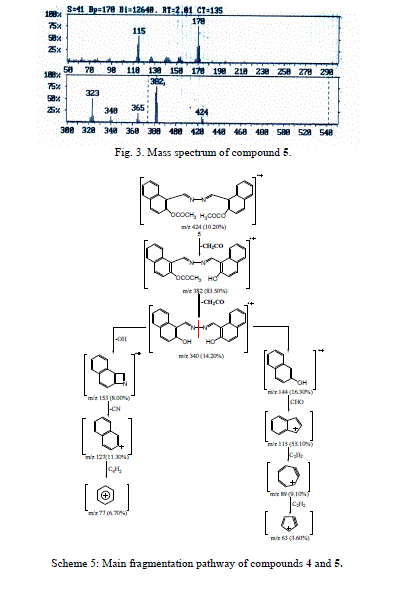
| Accordingly, the same molecular ion of m/z 340 fragmented via the pathway B to give a stable ion at m/z 170, and it underwent further loss of cyano group (CN) and gave peak at m/z 144. This fragmentation led to ions m/z 115, 89 and 63 by losing formyl group (CHO) and two molecules of acetylene, respectively. The molecular ion peak of compound 5 was observed at m/z 424 (Fig. 3). The loss of the ketene molecule (CH2CO) from the molecular ion peak at m/z 424 gave peak at m/z 382. The fragment ion of m/z 382 fragmented to give a peak at m/z 340, corresponding to the molecular ion of compound 4 by losing the ketene molecule. The fragment of m/z 340 broke further via a pathway similar to compound 4 (Scheme 5). |
 |
| 5-Bromo salicyladazine 11 |
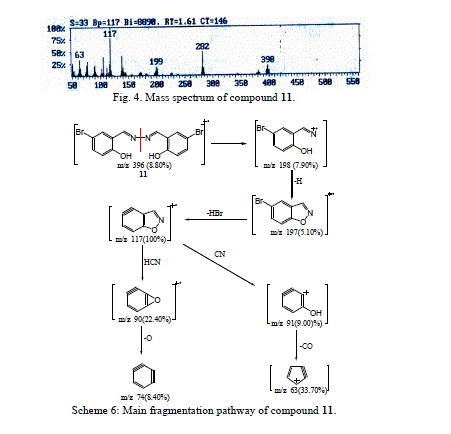
| The molecular ion peak of compound 11 was observed at m/z 396/398, corresponding to the molecular formula C14H10N2 Br2O2. From the study of mass spectra of the compound 11 (Fig. 4), it was found that the molecular ion peak for compound 11 fragmented to produce ion peak at m/z 198. The loss of hydrogen atom from the ion of m/z 198 gave a peak at m/z 197. The fragmented ion of m/z 197 was broken to give a stable peak at m/z 117, which then lost hydrogen bromide molecule. The fragmentation of m/z 117 peak led to the ions at m/z 90 and m/z 74, respectively (Scheme 6). |
 |
| 3. Biological assay |
| 3.1. Antioxidant activity |
| The antioxidant of some 2-hydroxy-(or 2-acetoxy)-1-naphthaldazines (4 and 5), triacetyl thiosemicarbazon derivative 7 and 5-bromo salicyladazine 11 were determined by the scavenging of synthetic radicals 2,2-diphanyl-1-picrylhydrazyl (DPPH) in polar organic sovent [13]. Methanol solutions of the test compounds were prepared. Absorbance measurements were recorded immediately with a Milton Ray spectronic 201 UV-visible spectrophotometer. The decrease in absorbance at 515 nm was determined continuously, with data being recorded at 1 min intervals until the absorbance stabilized (16 min). Tocopherol was used as a reference standard at the same concentration of used tested compounds. The absorbance of the DPPH free radical with antioxidant was also measured as control and 95% methanol was used as blank. All the determinations were performed in three replicates and average. % scavenging of the DPPH free radical was measured using the following equation; |
| % DPPH radical-scavenging = [(Absorbance of control-Absorbance of test sample) /(Absorbance of control)] ×100 |
| Tested samples had been submitted for qualitative evaluation of the antioxidant activity. The provided samples had different antioxidant activity using DPPH radical scavenging method as shown by the following table 1. |
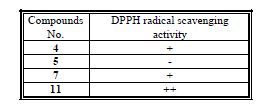 |
| (-): No activity (+): Weak activity (++): Moderate activity |
| (+++): Good activity (++++): Strong activity |
| The results indicated that compounds 4 and 7 were observed weak active, and also, that compound 11 was observed moderate active. The compound 5 was found to be inactive. |
| 3.2. Anticancer activity |
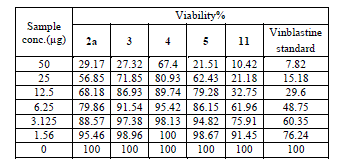
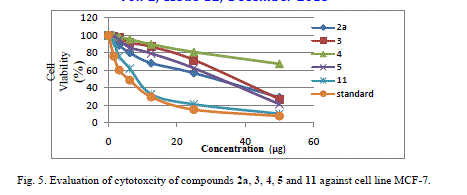
| Cytotoxic and antitumor activity of prepared compounds 2a, 3, 4, 5, 7 and 11 were evaluated against cell lines MCF-7 and HePG-2 according to the method of Mosamann and Vijayan et al [14,15]. The drug vinblastine was used as standard. Inhibitory activity against breast carcinoma cells (MCF-7 cell line), and hepatocelluar carcinoma (HePG-2 cell line) was tested by using different concentrations of the samples (50, 25, 12.5, 6.25, 3.125, and 1.56 μg), and cell viability (%) was determined by colorimetric method. The 50 % inhibrtory concentration (IC50) of the MCF-7 cell line was calculated from table 2 and (Fig. 5). The 50 % inhibitory concentration (IC50) of the HEPG-2 cell line was collected from table 3 and (Fig. 6) |
 |
 |
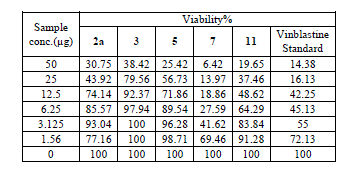 |
| The IC50 Values is the concentration that induces 50% growth inhibition compared with untreated control cells. |
| MCF-7: Human breast carcinoma cell line. |
| HEPG-2: Human hepatocellular carcinoma cell line. |
| Compound 7 was found to be more active than standard antitumor drug Vinblestine against HePG-2 cell line and it can be used as drug. In comparison with standard antitumor drug Vinblestine, Compound 11 was found to be more active against MCF-7 and HEPG-2 cell lines than the other prepared compounds |
III. EX PERIMENTAL |
| NMR spectra were recorded on general electric QE3000 instrument and chemical shifts were given with respect to TMS. IR spectra were recorded on PerkinElmer 1420 spectrometer and Biorad FTS7 (KBr). Mass spectra were recorded on a GC/MS with CI (chemical ionization) and a Hewlett-Packard MS-Engine thermospray and ionization by electron impact at 70 eV. The accelerating voltage was 6 kV; the temperature of the ion source was ~ 200 °C and the emission current ~100 mA. Microanalyses were conducted using an Elemental analyzer 1106. Melting points were determined on a Reichet hot stage. |
| 3-Acetylbenzo coumarin 2a and 3-Ethoxycarbonylbenzocoumarin 2b |
| A mixture of 2-hydroxy-1-naphthaldehyde (0.01 mol) and active methylene (such as ethyl acetoacetate and diethylmalonate (0.01 mol) in presence of piperedine (1 ml) was fused in a hot plate at 60 °C for 1h. The reaction mixture was cooled and acidified with hydrochloric acid (2N). The Crude product was filtered off, washed with water, dried purified by recrystallization with ethanol to give 2. |
| Compound 2a as yellow crystals; yield 76%, m.p. 189°C. IR spectrum (KBr), ν/cm-1:1730, 11695(C=O) of the pyrane and ketone), 1605,1585(C=C), 1250,1130(C-O) cm-1. 1H-NMR (DMSO-d6): δ, 1.31-1.34 (t, 3H, CH3), 4.29-4.32 (q, 2H, OCH2), 7.31-8.61 (m, 6H, Ar-H), 9.30 (s, 1H, H-pyran) ppm. MS: m/z (%):239 (M++1, 27.80), 238 (M+, 27.80), 224(27.80), 223(44.40), 222(38.90), 195(33.30), 191(22.20), 190(27.80), 168(38.90), 167(38.90), 160(16.70), 159(27.80), 158(27.80), 157(27.80), 156(33.30), 144(22.80), 142(27.80), 141(22.20), 140(27.80), 139(50.00), 128(44.40), 116(5.60), 115(22.20), 93(50.00), 92(27.80), 84(88.90), 83(83.80), 82(55.60), 81(33.30), 75(33.30), 74(33.30), 72(55.60), 71(44.40), 70(88.90), 69(66.70), 67(55.60), 66(33.30), 65(77.80), 64(77.80), 63(50.00), 61(33.30), 60(66.70), 59(50.00), 58(27.80), 57(61.10), 55(100), 53(44.40), 51(38.90). for C15H10O3 wi(calc.): 75.63% C, 4.20% H.; wi(found): 75.46% C, 4.01% H. |
| Compound 2b as pale yellow crystals, yield 75%, m.p. 142°C, IR(KBr): 1755, 1725 (CO of ester and pyran ring ), 1615, 1605, 1585 (C=C), 1295, 113, 1020(C-O) cm-1 . 1H-NMR (CDCl3): δ, 11.31-1.34 (t, 3H, CH3), 4.29-4.32 (q, 2H, OCH2), 7.31 – 8.61 (m, 6H, Ar- H), 9.30 (s, 1H, H-pyran) ppm. MS: m/z (%): 269 (M++1, 12.60) , 268 (M+, 63.10), 267 (M+-1, 5.70) , 241(1.80), 240(10.30), 239(5.20), 239(5.20), 225(1.70), 224(8.50), 223(51.60), 222(5.80), 197(13.30) , 196(83.10), 195(22.80), 194(6.60), 169(5.80), 168(39.30), 167(6.20), 166(4.20), 152(2.80), 151(6.80), 150(3.20), 140(19.60), 139(100), 138(27.20), 137(9.80), 128(3.4), 127(5.30), 126(3.20), 115(2.00), 114(3.60), 113(11.00), 112(11.00), 111(11.30), 99(2.60), 98(7.20), 97(4.80), 89(13.80), 88(6.30), 87(10.60), 86(9.20), 77(4.30), 76(3.90), 75(8.00), 74(7.80), 70(23.70), 69(23.20), 68(6.80), 65(4.40), 64(7.40), 63(29.60), 62(13.20), 51(6.30), 50(7.20) for .C16H12O4 wi(calc): 71.64% C, 4.47% H; wi(found): 71.46% C, 4.38% H. |
| 1,2-Bis[(o-hydroxy-1-naphthaldiene)amino]-ethane 3 and 2-hydroxy-1-naphthaldazine 4 |
| A mixture of 2a and /or 2b (0.01 mol) and strong base (namely, ethylenediamine and hydrazine hydrate (0.01 mol) in ethanol (30 ml) was heated under reflux for 2hr. The reaction mixture was cooled and poured into ice-diluted hydrochloric acid (1N). The product formed was collected by filtration, washed with water, dried and purified by recrystallization with dimethylformamide to give 3 and 4. |
| Compound 3 as yellow crystals; yield 61%, m.p. 389°C. IR(KBr): 3454(OH), 1632(C=N), 1615, 1605, 1585(C=C), 1259, 1039(C-O)cm-1. 1H-NMR (DMSO-d6): δ, 3.20 (s , 4H, 2X- CH2-N), 7.23-8-51 (m, 12H, Ar-H) , 9.93 (S, 2H, 2X=CH), 12.83 (s, 2H, 2X-OH) ppm. 13C-NMR (DMSO-d6): δ, 169-10(C-OH), 150.03 (C=N), 131.49, 129.22, 128.89, 126.64, 126.20, 122.81, 120.02, (C-aromatic), 33.25(NCH2) ppm. MS: m/z(%): 369(M++1,8.20), 368(M+,26.30), 367(M+-1,13-60), 225(1.40), 223(1.20), 212(2.70), 211(2.20), 210(1.40), 199(6.20), 198(16.60), 197(25.00), 196(16.30), 195(4.40), 185(15.50), 184(100), 183(51.60), 182(61.60) 181(24.90), 172(10.90), 171(16.90), 170(41.70), 169(20.90), 167(16.20), 158(5.10), 157(31.80), 156(18.60), 154(12.30), 152(6.70), 144(6.80), 144(6.80), 143(4.90), 130(2.80), 129(11.60), 128(23.70), 127(24.50), 126(11.40), 116(6.50), 115(28.60), 114(15.50), 113(4.50), 103(2.20), 102(4.50), 101(5.80), 89(6.50), 88(4.20), 78(3.50), 77(11.40), 76(5.50), 75(5.90), 65(4.10), 64(2.10), 63(9.90), 62(6.30), 55(1.70), 54(6.70), 51(6.70), 50(5.50)for C24H20N2O2 wi (calc.) :78.26%C, 5.43%H, 7.61%N; wi (found) :78.05%C, 5.22%H, 7.51N. |
| Compound 4 as yellow crystals, yield 56%, m.p. 289°C. IR(KBr): 3465 (OH), 1633 (C=N), 1605, 1589(C=C) , 1233, 1035(C-O) cm-1. 1H-NMR (DMSO-d6): δ, 7.20-8.60 (m, 12H. Ar-H), 9.97 (s, 2H, 2xCH=N), 12-86 (s, 2H, 2X-OH) ppm. 13C-NMR (DMSO-d6): δ, 168.13 (C-OH), 150.24 (C= N), 131.50, 129.23, 128.90, 126.84, 126.23, 122.91, 120.21 (aromatic) ppm. MS: m/z(%): 341(M++1,4.50), 340(M+,30.50), 339 (M+-1,12.30), 323(16.20) , 171(29.70), 170(100), 169(36.40), 154(13.60), 152(9.70), 144(4.50), 143(10.40) ,142(8.40), 141(7.10), 140(3.90), 139(5.20), 127(5.80), 126(7.80), 117(1.90), 116(13.50), 115(47.40), 1 14(29.00), 113(9.70), 98(4.50), 97(2.60), 89(9.10), 88(7.10), 87(5.20), 85(5.80) ,77(6.50), 73(8.40), 70(7.10), 69(5.20), 65(7.10), 64(6.50), 63(13.6), 62(7.10), 57(5.80), 55(6.50), 51(12.3), 50(10.40),for C22H16N2O2 wi (calc.) : 77.65% C, 4.70% H, 8.24% N, wi (found) : 77.42%C, 4.52%H, 8.01%N. |
| 2- hydroxyl-1-naphthaldehyde thiosemicarbazone 6 |
| A mixture of 2b (0.01 mol), thiosemicarbazide (0.02 mol) and anhydrous potassium carbonate (0.03 mol) in ethanol (50 ml) was heated under reflux for 3hr. The reaction mixture was cooled and poured into ice-diluted hydrochloric acid (1N). The resulting solid was filtered off, washed with water, dried and purified by recrystallization with ethanol to give 6 as pale yellow crystals, yield 63%, m.p. 272°C. IR(KBr): 3551-2981 (br.OH), 3423, 3165(NH2), 3285(NH), 1625(C=N), 1326(C=S), 1215, 1039(C-O) cm-1. 1H-NMR (DMSO-d6): δ, 7.12 (s, 2H, NH2), 7.312-8.41 (m, 6H, Ar-H), 9.01(s, 1H, CH=N), 10.46(s ,1H, NH), 11.38(s , 1H, OH) ppm. 13C-NMR (DMSO-d6): 192.32(C=S), 146.38(C-O), 139.20(C=N), 130.33, 129.28, 127.71, 126.22, 124.69, 123.38, 122.62, 118.24(C-aromatic) ppm. MS: m/z(%): 246 (M++1, 1.20 ) , 245 (M+,9.7), 224(53.80), 223(30.80), 171(53.80), 150(38.50), 149(69.20), 141(38.50), 140(46.20), 116(38.50), 115(100), 102(15.40), 101(61.50), 98(23.10), 97(76.90), 92(30.80), 41(46.20), 84(46.20), 83(46.20), 82(38.50), 78(53.80), 77(15.40), 73(23.10), 63(15.40), 60(76.90), 59(38.50), 58(61.50), 56(23.10), 52(75.90), 51(92.30).for C12H11N3OS. wi (calc.):58.77%C, 4.34% H, 17.14% N, wi (found) 58.57%C,4.34%H,17.02%N. |
| 2-acetoxy-1-naphthaldazine 5 and 4-(2-acetroxy naphthaldazine-1-yl)-1,3-diacetylthiosemicarbazide 7 |
| A solution of 4 and 6 (0.01 mol) in acetic anhydride (30 ml) was heated under reflux for 2 hr, then cooled and poured into ice-water. The solid obtained was filtered off, washed with water, dried and purified by recrystallization with ethanol to give 5 and 7. |
| Compound 5 was pale yellow crystals, yield 71%, m.p.171°C. IR(KBr):1756(CO ester), 1628 (C=N), 1605, 1586(C=C), 1285, 1151, 1031, (C-O) cm-1. 1H-NMR (DMSO-d6): δ, 2.30-2.51 (br. s, 6H, 2XCOCH3), 7.43-8.165 (m, 12H, Ar-H), 9.35(s, 2H, 2XCH=N) ppm. 13C-NMR (DMSO-d6): δ, 169.93 (C=O), 158.19(C-O), 150.28(C=N), 131.51, 129.29, 128.42, 126.88, 126.26, 122.92, 120.24(C-aromatic), 21.42(CH3) ppm. MS: m/z(%): 425 (M++1,15.00), 424(M++18.20), 383(2.60), 382(8.50), 381(4.00), 367(1.80), 366(5.90), 366(5.90), 365(20.00), 364(7.30), 341(4.30), 340(13.00), 339(3.50), 324(12.90), 323(49.90), 322(11.60), 321(7.80), 295(2.70), 294(0.90), 268(1.20), 212(1.30), 211(1.30), 197(1.00), 196(1.60), 195(2.40), 183(2.50), 182(1.10), 172(7.10), 171(34.10), 170(100), 169(31.70), 168(6.60), 154(11.20), 153(8.00), 152(9.30), 145(2.10), 144(6.30), 143(8.50), 142(7.80), 141(9.20), 140(8.80), 139(4.60), 128(7.00), 127(11.30), 126(7.80), 116(7.40), 115(55.10), 144(35.60), 113(10.00), 90(1.20), 89(6.20), 88(7.60), 87(4.70), 77(6.70), 76(3.60), 75(4.40), 65(2.80), 64(3.40), 63(8.00), 62(3.20), 57(4.00), 56(2.00), 55(2.50), 51(5.10), 50(13.70).for C26 H20N2O4 wi (calc.):73.58% C, 4.72% H, 6.60% N, wi (found):73 .37% C, 4.54% H, 6.52 N% |
| Compound 7 as pale yellow crystals; yield 68%, m.p. 164°C. IR(KBr):1745(CO of ester) 1698-1687(br.CO of ketone), 1629(C=N),1610, 1605,1589 (C=C), 1339(C=S), 1205, 1037 (C-O) cm-1. 1H-NMR (DMSO-d6): δ, 2.31-2.44 (br. s, 6H, 2XNCOCH3), 2.51(s, 3H, OCOCH3), 7.30-8.41 (m, 6H, Ar-H), 8.99 (s, 1H, CH=N), 10.42(s, 1H, NH) ppm. 13C-NMR (DMSO-d6): δ, 143.47 (C=S), 172.67, 170.10, 169.66(C=O), 146.32 (C=O), 138.00(C-N), 130.36, 129.38, 127.77, 126.29, 124.78, 123.48, 122.65, 118.25 (C-aromatic), 23.09, 21.60, 21.49 (3XCH3) ppm. m/z(%): 372(M++1, 2.90), 371(M++11.30), 370(M+-1, 3.30), 329(6.40), 328(17.00), 327(5.60), 288(2.10), 287(7.50), 286(21.10), 285(3.90), 271(1.80), 270(8.40), 269(3.40), 268(1.40), 246(1.20), 245(8.30), 244(11.80), 243(10.50), 228(9.50), 227(11.10), 225(5.40), 214(1.90), 213(1.40), 212(3.50), 203(5.30), 202(5.00), 201(6.30), 200(7.30), 188(3.60),187(15.70), 186(5.90), 185(9.80), 171(10.80), 170(41.80), 169(100), 168(20.60), 157(3.90), 156(2.20), 145(3.20), 144(15.80), 143(4.10), 141(4.20), 140(4.50), 129(4.10), 128(12.30), 127(10.50), 119(30.30), 118(15.90), 115(22.70), 114(8.90), 102(8.40), 101(5.20), 100(3.60), 89(2.80), 88(2.40), 87(2.20), 77(4.00), 76(11.40), 75(6.20), 65(1.30), 64(0.90), 63(4.70), 51(2.20), 50(1.10). for C8H17N3O4S, wi (calc. ): 58.22% C, 4.58% H, 11.32% N; wi (found) : 58.07% C, 4.44% H, 11.13% N. |
| 5-Bromo-3- benzoylamino coumarin 9 and 3- Benzoylamino benzocoumarin 10 |
| A mixture of N-benzoylglycine (8, 0.1 mol), 2-hydroxyaldehydes (such as 5-bromo-2-bydroxy benzoldehyde and 2- hydroxyl-1- naphthaldehyde) (0.01 mol), fused sodium acetate (0.03 mol) and acetic anhydride (0.03 mol) was fused in a hot-plate for 5 min. The reaction mixture was heated on a water bath for 2hr, then cooled and poured onto water. The resulting solid was filtered off, washed with water, dried and purified by recrystallization with acetic and to give 9 and 10. |
| Compound 9 as yellow crystals; yield 73%, m.p. 265 °C. IR(KBr), 3225(NH), 1725, 1689 (C=O of pyrane and amide), 1610, 1605, 1593(C=C), 1215, 1037(C-O) cm-1. 1H-NMR (DMSO-d6): δ, : 7.32-7.97 (m, 8H, Ar-H), 8.58(s, 1H, Hpyrane), 9.78 (s, 1H , NH) ppm. m/z(%): 345 (M++2, 68.20), 344(M++1, 38.50), 343(M+,72.80), 337 ( 12.50), 336(11.20), 335(12.30), 334(11.40), 299(18.0), 297(23.80), 254(17.20), 253(63.80), 252(62.90), 251(63.30), 250(62.20), 226(52.90), 225(51.60), 224(52.50), 223(51.80), 199(8.02), 198(9.20), 196(7.20), 194(11.30), 183(7.00), 182(4.00), 181(11.50), 180(5.00), 172(7.00), 169(22.50), 167(22.30), 166(22.40), 155(8.00), 154(7.00), 152(11.20), 150(6.00), 149(13.00), 139(11.60), 131(11.00), 117(11.60), 116(11.30), 115(6.00), 106(8.30), 105(100), 104(20.40), 103(4.50), 91(11.40), 90(11.00), 89(12.80), 88(5.10), 84(97.20), 83(15.40), 78(2.80), 77(38.80), 76(12.30), 75(5.90), 74(2.80), 63(3.80), 62(4.50), 56(7.50), 55(5.70), 51(13.30), 50(10.50). For C16H10NBrO3, wi (calc.) :55.98% C, 2.91% H, 4.08% N; wi (found) 55.78% C, 2,79% H, 4.01% N. |
| Compound 10 as yellow crystals; yield 72%, m.p. 252°C. IR(KBr), 3227 (NH), 1729, 1697(C=O of pyrane and amide). 1612, 1605, 1595(C=C), 1225, 1037 (C-O) cm-1. 1H-NMR (DMSO-d6): δ,:7.01- 7.98(m ,11H, Ar-H), 8.59(s, 1H, Hpyrane), 10.31 (s ,1H, NH) ppm. m/z(%): 316((M++1,3.10), 315(M+, 15.20) , 314(M+-1,1.70), 287(0-90 ), 286(0.30), 268(0.60), 267(0.20), 223(0.50 ) ,212(0.80), 211(0.10), 196(1.00), 195(0.30), 183(0.30), 182(1.10), 172(0.40), 171(0.90), 168(0.80), 154(1.40), 153(1.80), 152(1.10), 128(1.10), 127(5.60), 126(3.40), 125(1.10) , 106(8.20), 105(100), 104(20.90), 78(3.80), 77(47.30), 76(11.10) , 75(2.80) , 65(0.30), 63(2.10), 62(1.00), 51(12.50), 50(4.90). for C20H13NO3, wi ( calc): 76.19% C, 4.13% H, 4.44% N; wi (found): 76.03% C, 4.01% H, 4.22% N. |
| 5- Bromo-salicyladazine 11 and 2- Hydroxyl-1-naphthaldazine 4 |
| A mixture of hydrazine hydrate (0.03 mol) and coumarin derivatives 9 and/or 10 (0.01 mol) in ethanol (50 ml) was heated under reflux for 2- 3hr, then cooled and acidified with diluted hydrochloric and (2%). The resulting product was collected by filtration washed with water, dried and recrystallised from benzene to give 11 and 4. |
| Compound 11 as pale orange crystal; yield 53%. m.p. 210°C. IR(KBr), 3495(OH ), 1635(C=N), 1613, 1605(C=C), 1285, 1037(C-O) cm-1. 1H-NMR (DMSO-d6): δ, 7.41-8.71 (m, 6H, Ar-H), 9.71(s, 2H, 2XCH=N), 11.36 (s, 2H, 2XOH) ppm. m/z(%): 400(M++4, 10.5), 399(M++3, 8.80), 398(M++2, 19.10), 397(M++1, 16.80), 396(M+, 8.80), 395(M+-1, 9.20), 383(4.20), 382(2.20), 381(8.00), 380(3.60), 379(3.30), 378(2.40), 345(1.90), 343(2.40), 285(4.30), 280(5.70), 238(1.50), 237(1.50), 227(4.60), 226(2.90), 225(4.00), 201(13.40), 200(8.70), 199(17.40), 198(7.90), 197(5.10), (196(2.50), 183(2.5), 182(2.50), 180(240), 173(6.20), 172(4.40), 171(7.10), 170(5.10), 157(2.70), 155(2.00), 146(2.80),145(7.10), 144(5.40), 143(7.50) ,140(16.00), 139(9.90), 138(40.40), 137(9.80), 119(10.20), 118(13.20), 117(100), 116(23.80), 113(6.70), 112(2.90), 111(16.40), 106(3.90), 105(39.30), 104(4.70), 103(5.80), 103(5.80), 102(15.20), 91(9.00), 90(22.40), 89(19.30),88(5.80),78(2.80),77(28.50), 76(16.00),75(22.50), 65(14.60), 64(16.10),63(33.70), 62(13.30), 53(11.40), 51(26.30), 50(21.90). For C14H10N2Br2O2; wi (calc.):42.42% C, 2.53% H, 7.07% N; wi (found):42.22% C,2.42% H,7.01% N. |
IV. CONCLUSION |
| In conclusions, we have described the preparation and biological activities of naphthaldazine derivatives, thiosemicarbazon and salicylaldazine derivatives which showed inhibitor activity against MCF-7 cell line and HEPG-2 cell line. The antioxidant activities of some prepared compounds were also evaluated. The biological data revealed that with slight modifications in the structure 7 can plan for the drug design. |
References |
|