ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
J.R.Gujarathi1, T.V. Rajale2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
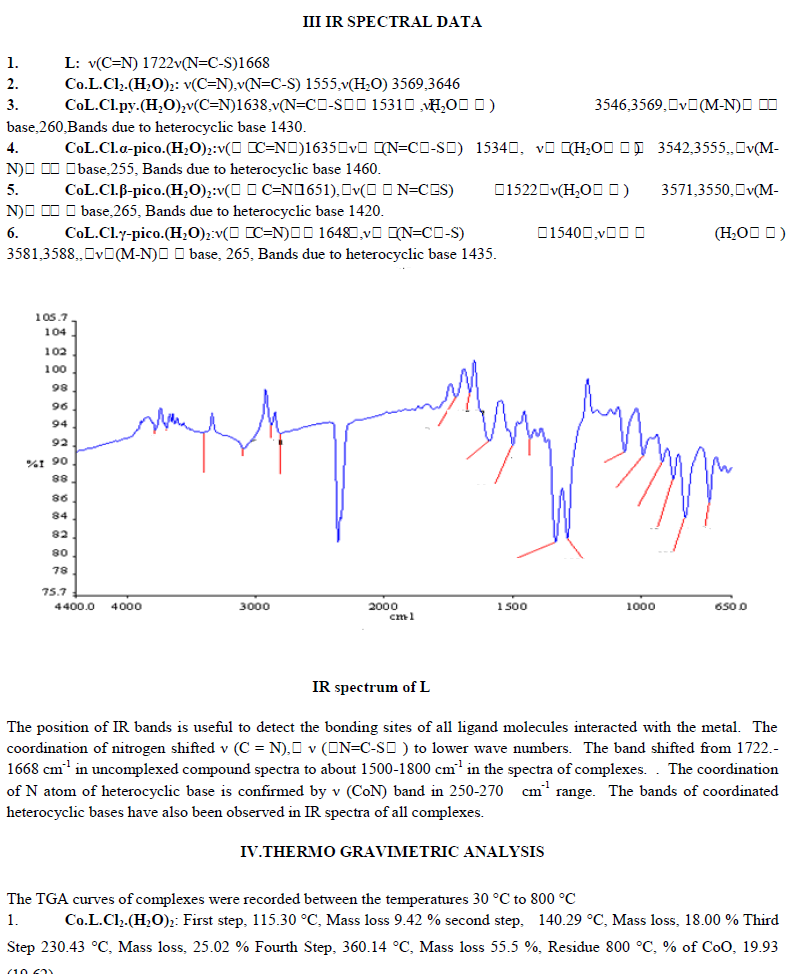
Six coordinate Co (III)-complex and adducts were synthesized by the reaction of CoCl2.6H2O with and 5-aminothiabendazole as chelate ligand in ethanol and heterocyclic bases like pyridine, bipyridine, α-picoline,β- picoline. The synthesized compound ATBZ was elucidated by elemental analysis, 13C, 1H NMR.The synthesized complex and adducts were characterized by elemental analysis,IR,TGA,magnetic measurement and conductivity. Screenings of antimicrobial activity of synthesized compounds were also carried out against different bacterial and fungal species such as Pseudomonas putida, Escherichia coli, Aspergillus Nigar, The synthesized compounds showed potent activity bacterial and fungal species
Keywords |
| 5-aminothiabendazole, CoCl2.6H2O, antimicrobial assay |
INTRODUCTION |
| The word 'cobalt' has been derived from the German 'Kobalt', from Kobold meaning 'globin', a word used by miners for the ore of cobalt [1]. Cobalt is required in the active centre of coenzymes called cobalamins. Cobalamins are pharmaceutical agents which are treated in pathologies arising from a lack of vitamin B12 [2]. In 1952 [3] the biological activity of cobalt complexes was reported. There has been interest in Co (III) complexes of bidentate mustards, which appear to act as hypoxia-selective agents [4]. Some Co (II) complexes have been found to be active against leukaemia, lymphoma cell lines [5] and bacterial strains [6]. Moreover Co (II) complexes possess in vivo insulin-like properties [7], antifungal [8] and antioxidant activity [9]. Dixit et al. have reported that, the Co (II) octahedral complexes exhibited good activity against both Gram (+) and Gram (-) bacterial strains but not higher than the free ligand alone [10]. |
| Complexes of Cobalt in +2 and +3 oxidation states have been prepared and investigated with focus on the reactivity of the metal ions in the transmethylation reaction and reversible absorption of molecular oxygen [11, 12]. Planer Cobalt (II) complexes of Schiff bases and related ligands have been used as catalysts for activation of molecular oxygen [13-18]. The study of Co (II) complexes of thiosemicarbazones is important because of their antitumour [19], antimicrobial [20] and electrical properties [20, 21]. |
| Sulfur and/or nitrogen heterocycles occur in the nature in the form of alkaloids, vitamins, pigments and as constituents of plant and animal cells. The utility of thiazoles in curative treatment has been firmly established. They exhibit antibacterial, anti-hypertensive, anti-anginal, anti-arrhythmetic, anti-histaminic, narcotic antagonist activities [21]. Thiazole nucleus is found in many antibiotics and vitamins. |
| The benzimidazole compounds are important group of fungicides. They have pronounced ability to control a large number of fungal diseases. Benomyl, thiabendazole and thiophanate methyl are examples of this fungicide class. |
| Because of their systematic activity, they can help to control some diseases after infection due to their activity. Benzimidazole fungicides are used to prevent post-harvest rots and in soil-drench treatments [23]. |
| The fungicidal properties of 2-(4-thiazolyl)-1H-benzimidazoles in plants have already been reported with protective and curative action. It is used to control of Aspergillus, Botrytis, Ceratocystis, Cercospora, Colletotrichum, Corticium, Diaporthe, Diplodia, Fusarium, Gibberella, Gloeosporium, Oospora, Penicillium, Phoma, Rhizoctonia, Sclerotinia, Septoria, Thielaviopsis, Verticillium spp., etc.[24] in asparagus, avocados, bananas, barley, beans, cabbage, celery, chicory, cherries, citrus, cotton, some cucurbits, flax, mangoes, mushrooms, oats, onions, ornamentals, pawpaws, pome fruit, potatoes, rice, soyabeans, strawberries, sugar beet, sweet potatoes, tobacco, tomatoes, turf, vines and wheat. Also used for control of storage diseases of fruits and vegetables and for control of Dutch elm disease. It is commonly used as an anthelminitic in human and veterinary medicine too [25]. |
| 5-Aminothiabendazole (ANTBZ) acts as both acid and base, thus it is possible to make compounds which are neutral, cationic or anionic in nature, as well as report biological activity of metal complexes. The potential N, N’- donor chelating agent are quite rare. In present paper we report synthesis and characterization of derivatives of 5- Aminothiabendazole, and differentiate fungi toxic activity with those of nitrothiabendazole. |
| Benzimidazole and many of its derivatives exhibit a variety of biological actions, including antibacterial, antiviral, anticancer and antifungal activity [26]. Benomyl, thiabendazole and thiophnate methyl are main examples of this fungicide class. Because of their systematic activity, they can help to control some diseases after infection. |
| In present work 5-Aminothiabendazole,is selected as chelte ligand because of structural similarity to chelating agents such as 2,2’ bipyridine and 1,10 phenanthroline. |
MATERIALS AND METHODS |
| Thiabendazole (A.R.Grade), Cobalt Chloride (A.R.Grade), zinc dust, methanol, formic acid, chloroform, sodium bicarbonate, super saturated solution of NaCl (A.R.Grade). |
Synthesis of Thiabendazole to 5-Nitrothiabendazole |
| Ice cold conc.H2SO4 was added to thiabendazole with constant stirring .The reaction mixture was warmed at 50º C for 10 min. till thiabendazole dissolved completely. In ice bath below - 4º C. Nitrating mixture (ice cold 1.5 ml conc.H2SO4 and 10.2 ml of conc. HNO3) was added with constant stirring. After complete addition the reaction mixture was kept aside for 45 min (i.e. at R.T.25º C). The reaction mixture was then warmed at 85-90º C for 90 min. The reaction mixture was then cooled at room temperature. Crushed ice was then added with constant stirring, very faint yellowish white precipitate was then separated out. Sodium bicarbonate was then added to it till the effervesces of CO2 completely stopped and precipitate became neutral. The precipitate was then filtered off, washed with water and finally with diethyl ether and dried under IR lamp. |
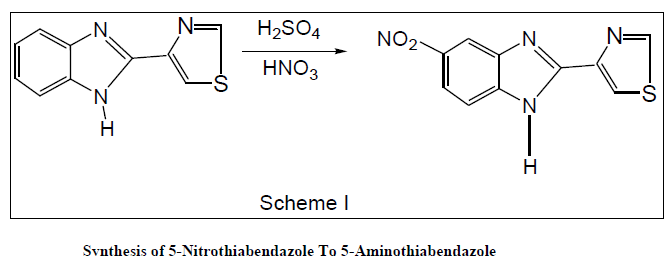 |
| Methanol was added to homogeneous mixture of nitro thiabendazole and Zn dust. Formic acid was then added slowly with constant stirring. The solution was filtered. The filtrate was then warmed to evaporate the organic solvent completely. Few drops of chloroform and hot supersaturated solution of NaCl were added to remove the formic acid completely. Sticky residue formed washed to convert to the powdered residue, filtered, dried under IR lamp and stored in vacuum. |
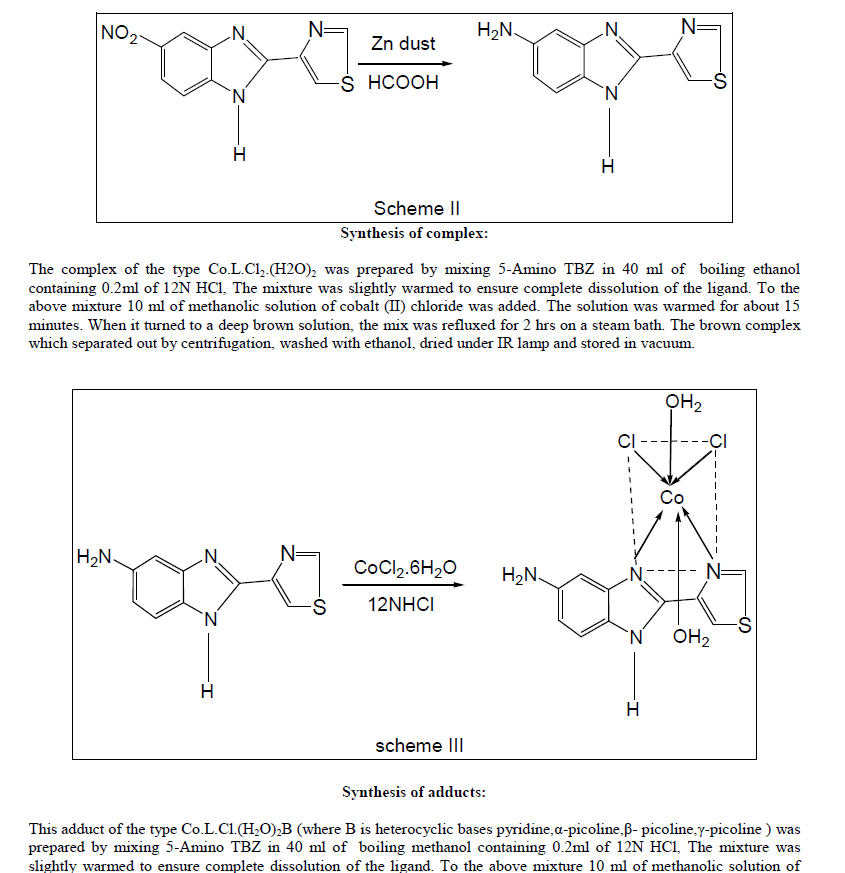 |
| cobalt (II) chloride and heterocyclic bases in the ratio 1:1:1 in ~25 ml of methanol. The mix was refluxed for 2 hrs on a steam bath. The brown adducts which separated out by centrifugation, washed with ethanol, dried under IR lamp and stored in vacuum. |
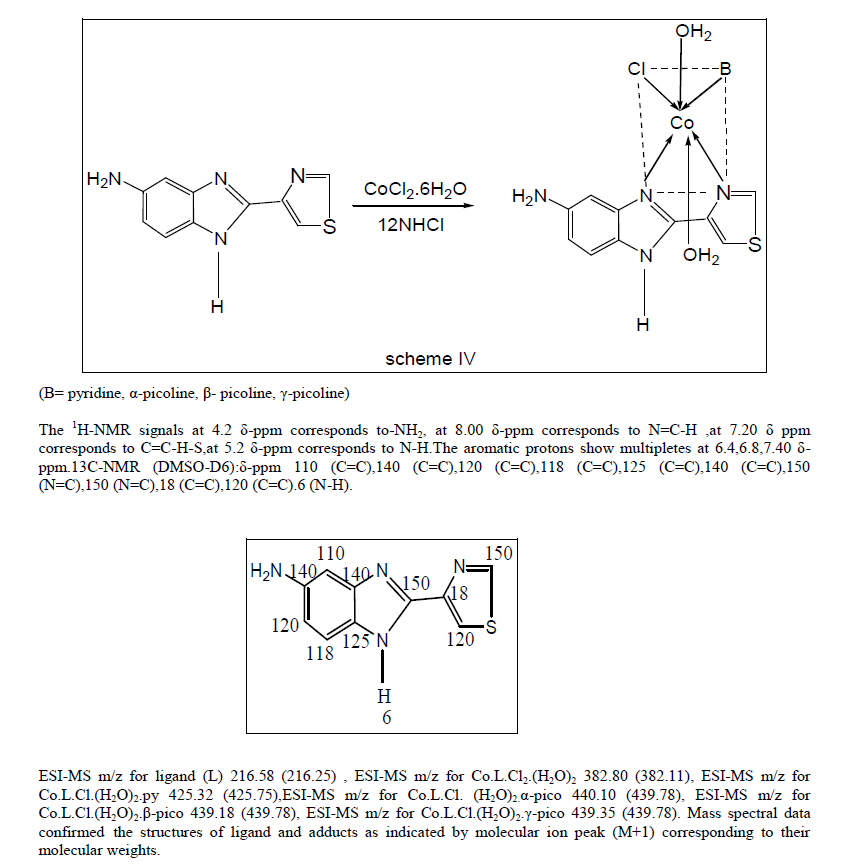 |
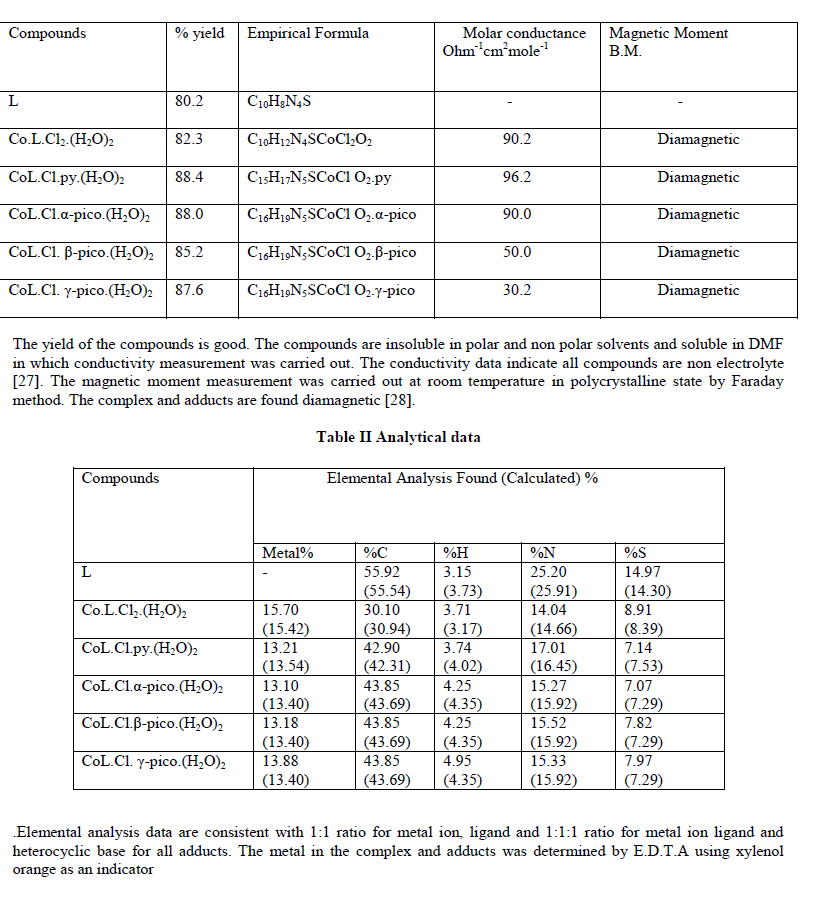 |
 |
 |
 |
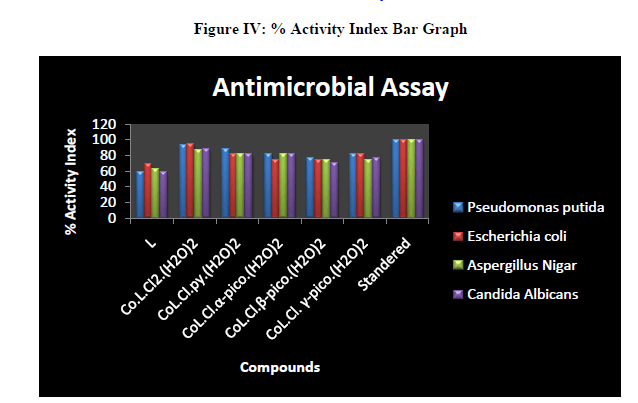 |
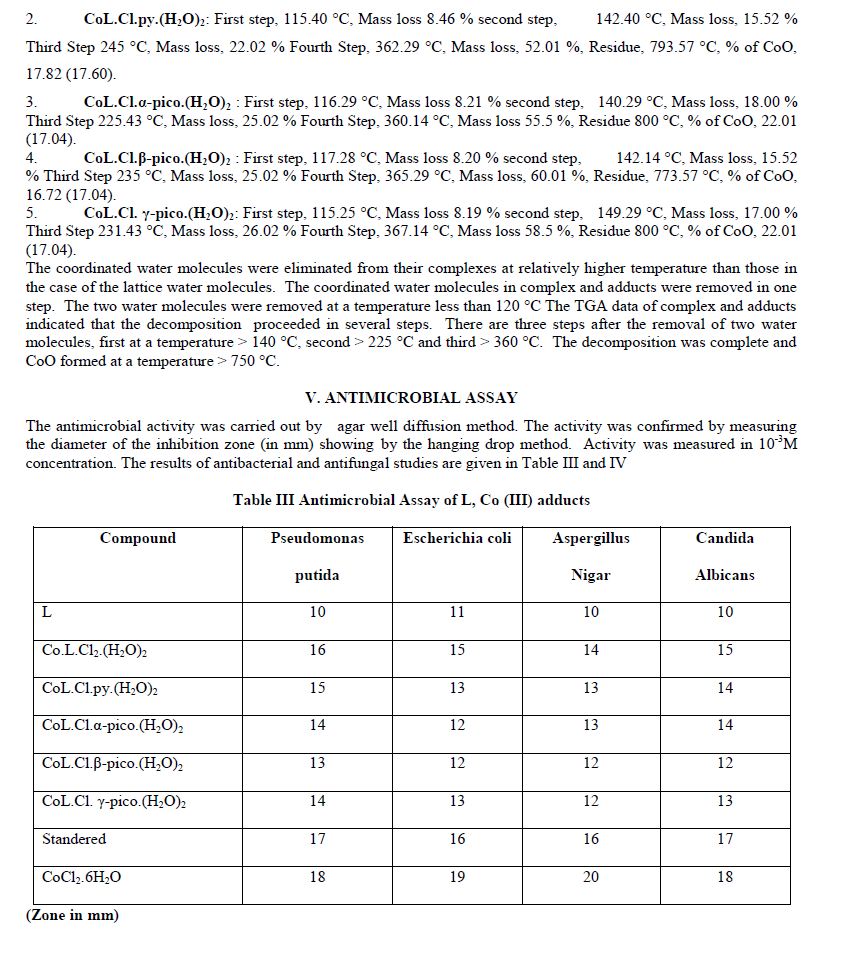
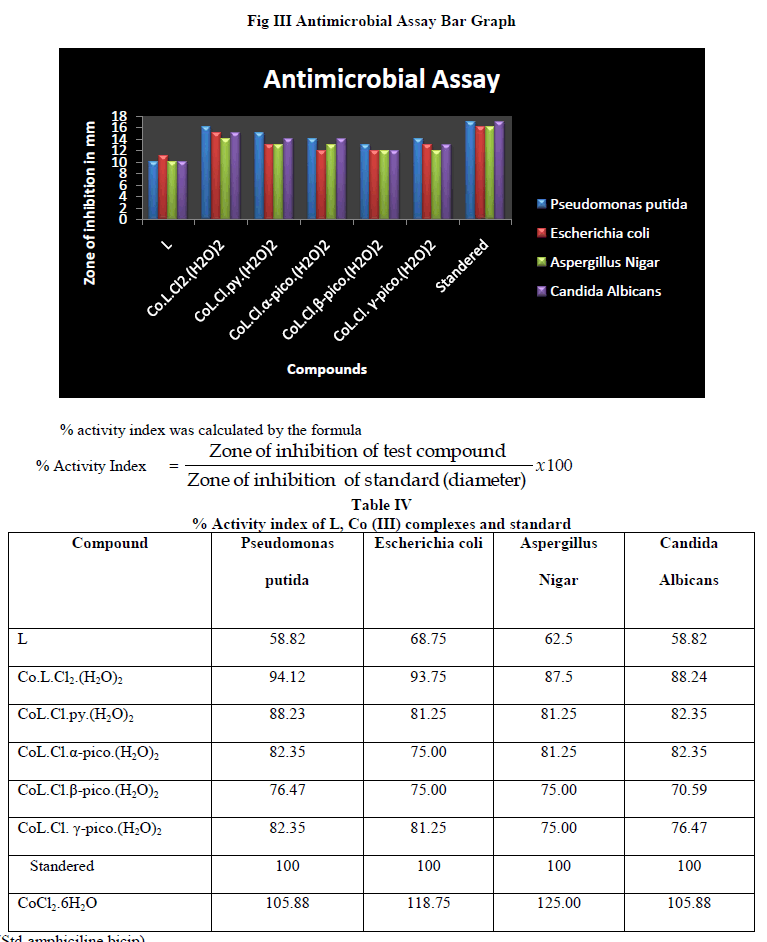
| The complex and adducts showed maximum activity against bacterial and fungal species than free ligand. The Co L.Cl2(H2O)2 exhibited high activity against all the bacterial and fungal species. Thus the coordination of metal ion to ligand is responsible for high biological activity. The most probable reason for this difference is chelation which reduces the polarity of the central metal atom because of the partial sharing of its positive charge with donor groups and possible π-electron delocalization within the whole chelating ring. As a result of this, the lipophilic nature of the central metal atom increases, which favors the permeation of the complexes through the lipid layer of the cell membrane [29]. |
| All spectral characterizations confirmed that the complex and adducts have octahedral geometry, with 5- aminothiabendazole acting as NN bidentate ligand and N–atom of heterocyclic base occupying the fourth coordination site about the Co (III) atom. The TGA curves indicated coordinated water molecules in complex and adducts. |
References |
|