ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Sreehari Sastry1*, M.V.V. K. Srinivas Prasad2, B. Rupa Venkateswara Rao3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
PbO-P2O5-ZnO-V2O5 glasses composed of different alkali oxides are prepared. The glasses are characterized by XRD, FTIR, EPR and UV-Visible spectral analysis. The functions of structural features and amorphous nature due to compositional changes are examined using XRD. FTIR spectroscopy is used to identify the presence of bond assignments and vibrational modes of phosphate in the system. Depolymerization of the phosphate network by the replacement of alkali oxide content in the glasses which are consisting mainly more of (PO4 3-) and (PO3 2-) units, is detected by the FTIR spectra. The spectral studies have exhibited an intense and broadband in the visible region which is related to VO2+ ions in a ligand field of C4v symmetry. The importance of bridging oxygen in improving the optical properties is studied.
Keywords |
| Phosphate Glass; FTIR; UV-Vis and EPR spectra; Bonding properties |
INTRODUCTION |
| Vibrational spectroscopic studies (FTIR) for vitreous P2O5, are useful to distinguish bridging and terminal oxygen atoms [1-2]. Electron paramagnetic resonance (EPR) is already proved to provide answers to significant questions about structure by showing the influence of electron spin–spin interactions between paramagnets. Thus, an investigation on the relationship between the composition and properties of these materials is seen to be important. Phosphate glasses have attracted considerable attention in recent years due to their nonlinear optical properties. Oxide glasses with large non-linear refractive index and non-linear absorption coefficient are used for optical fibre telecommunication and for non-linear optical devices such as ultra fast optical switches, power limiters, real time holography, self-focusing, white-light continuum generation and photonic applications [3-5]. As an extension of work [6,7], the present paper is to study the properties of VO (II) as dopant in Pb3O4-M2O-ZnO-P2O5 (where M=Li, Na and K) glasses (hereafter referred to as LPZ, NPZ and KPZ glasses) through various spectroscopic techniques like Electron Paramagnetic Resonance and Optical absorption and also to identify the importance of Pb (II-IV) in heavy metal combinations. This sort of information is useful to determine the microscopic insight of glasses for different nonlinear optical applications as well as for optical fibre amplifiers |
EXPERIMENTAL |
| The chosen materials are –Pb3O4, ZnO, V2O5, Na2CO3, Li2CO3, K2CO3and P2O5 of 99.9% Analar grade in this study. The host glass composition is taken as 10 Pb3O4 + 20 M2O+10 ZnO + 59.9 P2O5 + 0.1 V2O5 (where M = Li, Na and K). For each concentration the mixture is firstly sintered at 700 K after being thoroughly mixed by quench melting method. Then the sample is taken into a porcelain crucible and melted in an electric furnace at 1200 K for nearly 1hour. For quenching, the melt glass is formed at room temperature in air. Thereafter, the glass material is annealed at 550 K for 30 minutes. To ascertain amorphous nature of the glass, X-ray diffraction studies are performed through PHILIPS X'PERT PRO X-RAY diffraction system. The Fourier Transform Infrared spectra of these glasses are recorded on SHIMADZU 8201 PC FTIR Spectrophotometer in the range 4000-400 cm-1 using KBr pellets. EPR spectra are recorded at room temperature through JES-FA series X-band EPR spectrometer having 100 kHz field modulation. Optical absorption spectra of these glasses are recorded at room temperature through JASCO (V-530) spectrophotometer in UV-Visible region. |
RESULTS AND DISCUSSION |
| Figure 1 shows the XRD pattern of VO2+ doped alkali lead zinc phosphate glass system. As no significant crystalline peaks are recorded in the patterns, the amorphous nature of glass samples is confirmed here. |
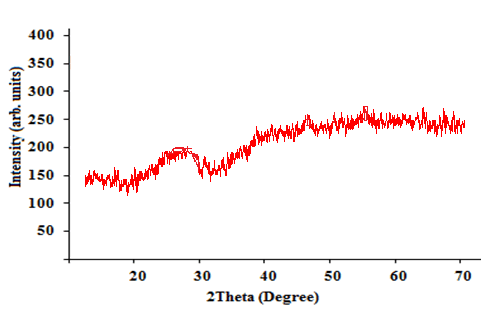 |
| Fig. 1 XRD spectrum of VO (II) ion in Alkali Lead Zinc Phosphate Glasses |
| The tendency of strong depolymerisation of the glass network by introducing alkali (Li, Na and K) oxides is also confirmed by the FTIR spectra (Fig. 2). The bands and their assignments are1230 cm-1 caused due to (P-O-) vibrations; 1060 cm-1 bands to PO4 3- fundamental vibrational mode; 880 cm-1 to asymmetric stretching vibrations of PO- P groups; 770 cm-1 a symmetric vibration to P-O-P chains and 600 cm-1 to bending vibration of the P-O-P bonds [8- 10]. FTIR study has, hence, established that alkali oxide entered into the network interstitially as a network modifier and later, depolymerisation is initiated by breaking of P-O-P linkages. |
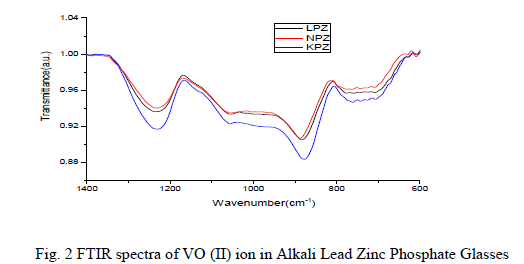 |
| Vanadium in tetravalent state exists almost exclusively as the vanadyl ion VO2+ which is formed when the V4+ with a single unpaired 3d electron is bound to oxygen by strong covalent bond. According to Selbin [11], this species is the most stable diatomic radical. Vanadium ions act as network modifiers but also as network formers depending on their concentration in the glass matrix. The ESR spectrum of V4+ in glasses has shown rich hyperfine structure due to the 51V nucleus (I = 7/2) which is easily observable in most systems at room temperature. The EPR spectra obtained at room temperature for alkali lead zinc phosphate glasses with a small content of V2O5 has shown a well resolved hyperfine structure typical for isolated vanadium ion in a ligand field of C4v symmetry, presented as vanadyl ion as shown in Figure 3. The spin-Hamiltonian is described in a way like |
| H = ïÃÂâ[ g|| BzSz + gïÃÂÞ(BxSx + BySy)] + A|| SzIz + AïÃÂÞ(SxIx+SyIy) (1) |
| here ïÃÂâ is the Bohr magneton while g||, gïÃÂÞ and A||, AïÃÂÞ are the components of the gyromagnetic tensor g and hyperfine structure tensor A, respectively. Bx, By and Bz are components of the magnetic field; Sx, Sy, Sz and Ix, Iy, Iz are the spin operators of the electron and the nucleus, respectively. |
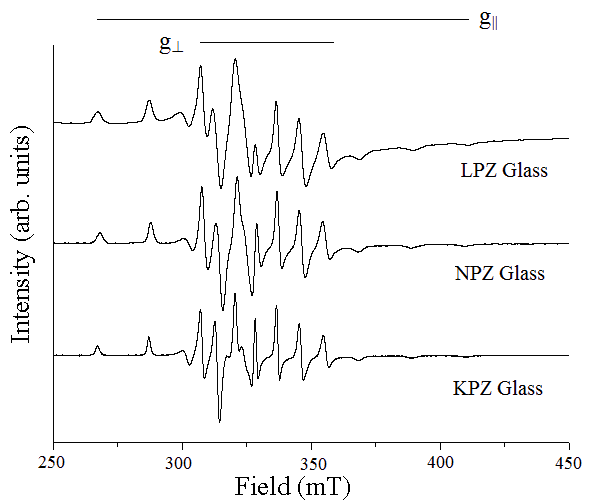 |
| Fig. 3 EPR spectra of VO (II) ion in Alkali Lead Zinc Phosphate Glasses |
| The spin-Hamiltonian and hyperfine values are evaluated from EPR spectra of vanadyl doped Alkali Lead Zinc Phosphate glass and shown in Table 1. An octahedral site with a tetragonal compression would give the values of g|| < gïÃÂÞ < ge. The present values of the spin-Hamiltonian parameters have agreed with the relation. Furthermore, the values of A- interaction matrices have the relation A|| > AïÃÂÞ for all those three samples. These observations have led to the conclusion that the paramagnetic V4+ ion in the glass has been present as vanadyl ion [VO(II)], in an octahedral environment of oxygens with tetragonal distortion where the vanadyl oxygen forms the apex V-O bond [12-14].The dipolar coupling constant (P) and Fermi-constant coupling parameter (ïÃÂë) are evaluated using the following formulae [15]. |
| A|| = P [(-4/7) – ïÃÂë + (g|| - ge ) + (3/7) ( gïÃÂÞ - ge )] (2) AïÃÂÞ = P [(2/7) – ïÃÂë+ (11/14) ( gïÃÂÞ - ge )] (3) where ge = 2.0023 |
| Table1: spin-Hamiltonian and hyperfine values of VO (II) ion in Alkali Lead Zinc Phosphate Glasses |
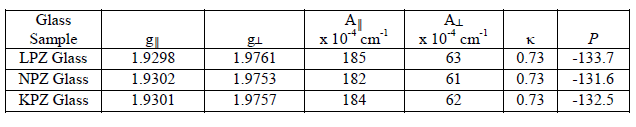 |
| The lower values of ïÃÂë have indicated the poor contribution of vanadium 4s orbital to the vanadyl bond in these glasses. It has also suggested that a hexa-coordinated geometry for the VO (II) ion in the complex of near octahedral (Oh) symmetry which is due to the weakness of the V-O bond in the vanadyl group caused by a strong axial perturbation arising from the sixth oxygen atom has coordinated in the transposition to the vanadyl oxygen [6,16]. The single d-electron of the VO2+ ion occupies the t2g orbital in the octahedral crystal field giving rise to 2T2g ground state. When excited, the electron occupies the upper eg orbital giving rise to 2Eg term. In ideal octahedral symmetry, only one band is expected to arose from the transition 2T2g ïÃÆà2Eg. However, VO2+ never exhibits an ideal octahedral symmetry but lowers to tetragonal (C4v ) or still lower symmetries like C2v because of the non-symmetrical alignment of the V=O bond. In C4v symmetry, 2T2g splits into 2B2 and 2E, where as 2Eg splitting into 2B1 and 2A1. Accordingly three bands are expected due to the transitions from the ground state (2B2) to the exited states 2E, 2B1, and 2A1). The general ordering of energy levels is as follows [15, 17]:2B2 < 2E < 2B1 < 2A1. Optical absorption spectra are shown in Figure 4. Here the recorded spectrum has revealed only two bands which are characteristic of VO (II) ion in tetragonally distorted octahedral site. The bands are attributed to the transitions 2B2 ïÃÆà2E and 2B2 ïÃÆà2B1 respectively. |
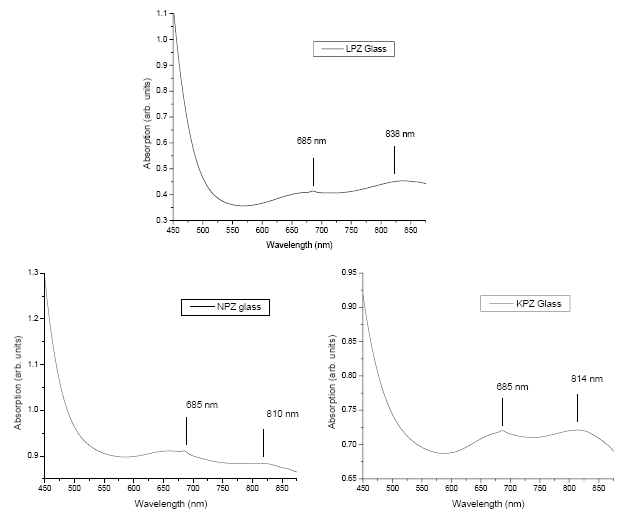 |
| The values of EPR parameters and the covalency degrees of the in- plane V-O ïÃÂó-bonds (ïÃÂâ*2) and of ïÃÂð-bonding (ïÃÂÃ¥ïÃÂð*2) with the equatorial ligands are equal to unity when the bonding is purely ionic. For a completely ionic ïÃÂð bond between the vanadium and vanadyl oxygen (ïÃÂÃ¥ïÃÂð*2) would be equal to unity. These coefficients represent value of 0.5 for a completely covalent bond. These parameters are evaluated with the help of Molecular Orbital scheme developed by Kivelson and Lee [16]. |
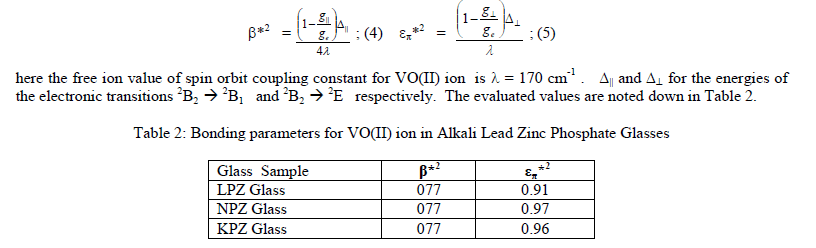 |
| Electron paramagnetic resonance and optical absorption studies have indicated covalent nature from both gvalues and bonding parameter values. LPZ glasses have more covalent nature when compared to others due to the presence of Pb4+ which developed the bridging oxygens. Therefore two states of Pb3O4 as Pb2+ and Pb4+ (2PbO + PbO2) are the major assets to have increased the lead concentration. When PbO entered the network, it is noticed to form PbO4 units in which lead is coordinated to four oxygens in a covalently bonded configuration. It is understood that lead did not reside in the glass network interstitially. For a constant content of P2O5, the substitution of ZnO by PbO has led to an increase in the network disorder. The changes in network structure, bonding nature, high amorphous character and increasing optical behaviour might have occurred due to not only by the presence of Pb4+ but also by alkali content which is deemed to be helpful in depolymerisation of phosphate network. Lithium is more effective than sodium and potassium in breaking the phosphate network because of its low ionic radius (Li<Na<K) and acting as modifier. Hence, it is unambiguous that the bridging oxygens caused by Pb4+ have developed Pb2+ - O – Pb4+ - O – Pb2+ and Pb–O-Zn chains linking the phosphate tetrahedral in a covalent bond configuration. The present spectroscopic studies have also revealed the same nature and indicating the decrease in network connectivity for this alkali lead zinc phosphate glass system. As lead partially participated in the network formation [18] and decomposed into nano scale separated entities, these entities have accounted for higher optical properties [18,19] for LPZ glass sample. Lead phosphate glasses possess enhanced optical non-linearity due to high polarizability of Pb2+ ions in glass matrices. Electrostatic interaction between transition metal ion and the highly excited Pb4+ ions is also known to cause this result [20]. |
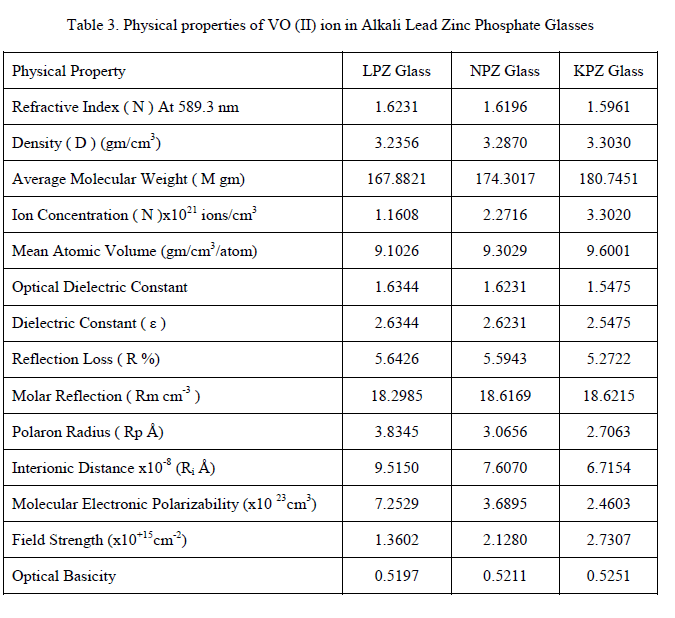
| The data of physical properties presented in Table 3 have indicated that the average molecular weight increases from LPZ to KPZ glass which influences density and other physical parameters. The increase in density for the glass system has shown the change in the structure of the glass with replacement of alkali content. It is evident that the mean atomic volume and optical basicity of the glasses increased with the replacement of alkali oxide. The theoretical value of optical basicity (ïÃÂÃÅth) reflects the ability of the glass to donate negative charge to the probe ion. The basicity parameter from Duffy and Ingram increased from glass to glass [21]. High optical basicity means high electron donor ability of the oxide ions to the cations [22]. Variations in physical properties are due to replacement of alkali oxides in different glasses around VO (II) ions |
 |
CONCLUSIONS |
| FTIR spectra have showed that the replacement of alkali oxides has depolymerised the phosphate glass network, due to breaking of P-O-P bonds and development of more new bonds. EPR studies have shown that gïÃÂÞ > g|| are representing tetragonally compressed octahedral site for the VO2+ ion. Thus, the inference is that vanadium enters the glasses as VO (II) ion. The value of (gïÃÂÞ - g||) indicates the tetragonal distortion existence in the present glass matrix. The optical absorption spectra also suggest the same with characteristic band transitions from 2B2 and the site symmetry for C4v. Only two transitions are observed. The FTIR results have supported similarly as both the results obtained from the EPR and optical absorption which inferred the major role of alkali in the formation of cross linking bands. The covalence degree of the in-plane V-O ïÃÂó-bonds (ïÃÂâ*2) and ïÃÂð-bonding with the vanadyl oxygen (ïÃÂÃ¥ïÃÂð*2) in the glass has indicated the covalent nature. The presence of heavy metal oxide combinations causes the occupation of most of the volume of the network to improve the optical nature of the glass and durability. Lastly, therefore, LPZ glass is more suitable glass matrix for enhanced nonlinear optical applications like dispersion. |
ACKNOWLEDGMENT |
| The authors gratefully acknowledge Prof. K.B. Mahalakshmi for his help, and University Grants Commission Departmental Research Scheme at Level III program No.. F.530/1/DRS/2009 (SAP-1), dated 9 February 2009, and Department of Science and Technology -Fund for Improving Science and Technology program No. DST/FIST/PSI – 002/2011 dated 20-12-2011, New Delhi, to the department of Physics, Acharya Nagarjuna University for providing financial assistance |
References |
|