ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Rajeev Kumar Sharma1, B. Tiwari2, J. S. Tomar3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In this present study a complex of transition mental cr+3 with amino acid present in egg albumin have been synthesized. To obtain metal carbon nanotube, this amino acid metal complex is decomposed at 8000 c in muffle furnace to give metal carbon nanotube. These metal carbon nanotubes are characterized by the scanning probe instruments such as SEM, XRD. The thermal stability has been studied using DTA, TGA, DTG.
Keywords |
| Cr (III), albumin, Albumin-metal-complex, SEM, XRD, DTA, TGA, DTG |
INTRODUCTION |
| Carbon nanotubes1 are molecular scale tubes of graphite carbon2. They are among the stiffest & strongest fibre known and having outstanding properties3. These outstanding properties3 motivate the scientist to develop new techniques to produce carbon nanotube4. To produce CNTs, a large no of carbon atom is required4. For this, proteins are used as key material, because proteins are the bio-polymers of amino acid5. Amino acid containing –NH2 & -COOH group. These group play important role to form metal complex. When these metal complex are decomposed at 8000 C then CNTs are formed6. |
II. SYNTHESIS BY ADVANCED METHOD |
| To prepare carbon nano tube, 1 gm normal metal salt solution of Cr+3 was prepared in alcohol. When this solution is mixed with egg albumin, a complex of egg albumin with chromium ion is formed. The metal albumin complex is decomposed in muffle furnace at 8000C. |
III. CHARACTERIZATION BY SCANNING ELECTRON PROBE INSTRUMENTS |
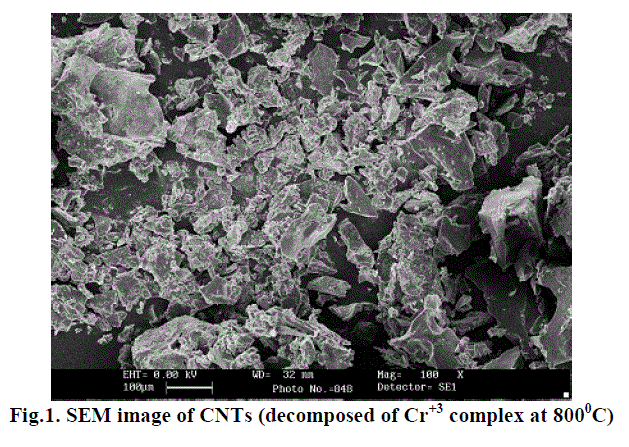
| A. SEM: scanning electron microscope is used to determine morphology & Size of material. Result of SEM is shown in fig. 1. From fig.1 it is clear that the thickness of CNTs 10000 nm with magnification of 100x. Fig.1 shows some white spots. These spot represents Cr+3 metal ion on the surface of CNTs. Which are responsible for electron transportation. |
 |
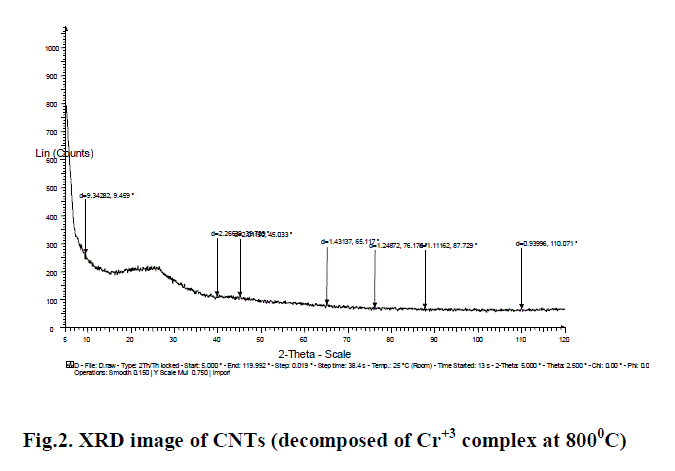
| B. XRD : The X-Ray Diffraction pattern is used to determined the structure of carbon nanotube. XRD pattern were recorded with Cu Ka radiation (1.54056 λ = A) in 2θ range from 15 to 80. Form analysis of the XRD report, we find different peak values, at different value of 2θ & d. for Example (2θ & d = 9.34282, 9.459, 2.26639, 39.739, 2.01150, 45.033, 1.43137, 65.117, 1.24872, 76.176, 1.11162, 87.729, 0.93996, 110.071), which show that the given sample is Multiwalled carbon nano tubes. The XRD report, as shown in fig. 2 was analyzed at IIT Roorkee. |
 |
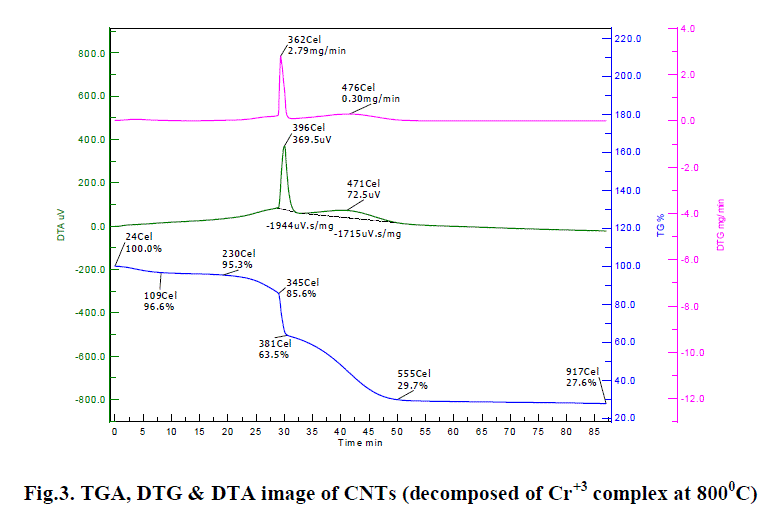
| C. DTA, TGA & DTG : Thermo gravimetric analysis of TGA is a type of testing performed on samples to determine changes in weight in relation to change in temperature. Such analysis tells that on a high degree of precision in three measurements. i. e. weight, temperature, and temperature change. The TGA thermograph (from Fig. 1) predicts 72.4 % mass decomposed from 100% - 27.6%, when temperature increase from 24-917 Cel. It becomes stable at 917 cel & stable amount of CNTs is 27.6%. After this decomposition does not occur. It proves the thermal stability of CNTs. From DTA graph (from Fig. 1), it is clear that decomposition stats at 396 Cel & 369.5 μV and again decreases as voltage decreases. There after increases on increasing voltage at 471 Cel. Stability of CNTs increases after 471 Cel & 72.5 μV. DTG graph (from Fig. 1) indicates that decomposition is maximum at 362 Cel and 2.79 mg/min and further at 476 Cel at 0.30 mg/min. and becomes stable after that. |
 |
III. RESULT AND DISCUSSION |
| The Carbon metal nanotube is formed which is found to be thermally stable7 even on increase in temperature and voltage. This also exhibits conductivity8, which shows the presence of unpaired electron. It is found to exhibit thermal conductivity also. |
V. CONCLUSION |
| Multi-walled carbon nano tubes have been prepared by advanced Chemical method. From SEM image metal ion presence is predicted on the surface of CNTs. The XRD studies confirm the Multi-walled structure of CNTs. |
References |
|