ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Suraj Nakhate1, Vaibhavi Ughade2, Sandip Petare3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Chlorophytum is a species of about 200-220 genus of evergreen plants which is native to the tropical and subtropical regions of Africa and Asia. There are about 17 species of Chlorophytum recorded in India out of which 11 species are found to be growing in Maharashtra. In the present study it was found that Chlorophytum tuberosum regenerate from the auxillary bud as a explants inoculated on MS medium supplemented with BAP (0.5mg/L) for shoot induction, BAP+NAA (0.25mg/L+0.50mg/L) for callusing and IAA (0.25mg/L) root induction respectively. Result is efficient for mass propagation of Chlorophytum tuberosum to justify progressively increasing demand of Chlorophytum tuberosum.
Keywords |
| Chlorophytum tuberosum, Auxillary bud, MS medium, BAP, Callus induction. |
INTRODUCTION |
| Since times immemorial human beings have endeavored for good health and immortality. Yoga (Techniques to elevate the physical and mental status of an individual), and Ayurveda (use of medicaments to maintain health, longevity and vitality) have played a pivotal role. Plants have been used as an important source of medicine since ancient times and their products are being used for different purposes such as medicine, food, health care, agriculture, agrochemicals, pharmaceutical, etc. Chlorophytum borivilianum San. and Fern. (Family Liliaceae) is a traditional endangered perennial herbaceous |
| medicinal plant commonly known as ‘safed musli’ [1] [2]. These are rhizomatous herbs and roots fancied, often thick fleshy and tubers like [3]. The roots of C. borivilianum are a constituent of ‘Chyawanprash’ an outstanding rejuvenator. It is known as the Indian Ginseng, because of great therapeutic importance and its tubers are the major constituents of more than 100 Ayurvedic preparations. Roots are the economic parts and having aphrodisiac properties due to presence of steroidal saponins, viz., neotigogenin, stegmasterol, tokoregenin [4]. The extract and juice of C. tuberosum use as the adaptogen reducing stress, increasing antioxidant activity nitric oxide levels a male sex hormone and preventing sexual disfunctions. Traditionally, tubers are used in the treatment of rheumatism and the leaves as vegetable in various culinary preparations and also used for its aphrodisial properties in lack of libido male impotency, oligospermia[5][6] |
| According to report of World Health Organization (WHO), nearly 80 per cent of the people in developing countries consume traditional medicines for sustaining health and vitality. According to one estimate 20,000 to 35,000 species of plants are used as medicines, pharmaceuticals, cosmetics and neutraceuticals by different ethnic groups the entire world over [7]. |
| Many countries in the Gulf, Europe including USA has been major importers of the dry roots of safed musli for a very long time, for its use making of various herbal products. Year by year due to increasing natural irregularities in monsoon in India naturally grown safed musli is insufficient in compensating the increasing demand worldwide. |
| So that, in vitro propagation of plants holds a tremendous potential for producing high quality plant based medicines [8] Tissue culture techniques have played an important role in the breeding, production, and improvement of crops and medicinal plants. Micropropagation allows rapid production of high quality disease free uniform planting material irrespective of the season and weather [9]. Micropropagation helps to produce plantlets of uniform genetic material as well as uniform phytochemical constituents as parent elite plant from which explant was taken. Successful micropropagation of any plant species depends on explant selection and morphogenetic responses of explants including the organ that is to serve as tissue source, the physiological, genetical age of the organ and size of the explant [10]. Attempts have been made to develop in vitro propagation of safed musli [11] [12] where in some of the parameters have been worked out. However, in order to propagate the quality planning material from the selected elite plants, further studies were required to investigate the various factors which influence large scale multiplication and subsequent acclimatizationc |
| The transfer of in vitro raised plantlets to ex vitro conditions was one of the most critical factors in the micropropagation process and cause of higher production costs. Due to weak root system and nonfunctional stomata mortality often observed, a biological approach to reduce the stress of acclimatization and provide faster growth of plantlets was the establishment of arbuscular mycorrhizal (AM) fungi association. The VAM-Glomusaggregatum reduces the osmotic potential of plantlets. This response may be useful pre-adaptation for in vitro developed plantlets during transfer to the acclimatization stage [13]. |
REVIEW OF RESEARCH |
| Nurashikin, et al [14] developed a protocol for sterilization and in vitro development of shoot bud from safed musli (Chlorophytum borivilianum). C. borivilianum species is valuable for the dried fasciculate storage roots [1]. Tuberous roots of C. borivilianum possess immuno-regulatory and adaptogenic properties, anti-ageing health restorative and are used to cure of impotency, sterility and enhance male potency. So to fill the gap of demand and supply and to provide genetically uniform planting material from a known source, tissue culture is one of the most desirable options. By considering that safed musli is an endangered species and the availability of planting material is scarce, the use of tissue culture technique provides a rapid method to mass produce the plant. |
| Thakare, et al., [15] attempted in vitro induction and characteristics of callus of Chlorophytum tuberosum. The protocol for callus induction using suitable explants that can be used for micropropagation was developed. Juvenile explant consisting of leaf, tubers, and hypocotyl of in vitro germinated seed of C. tuberosum were used. Initiation of callus was highest during 68 days in leaves on MS media supplemented with 5 mg 2,4-D/L and 00 mg kinetin/L and 5 mg 2,4-D/L and 1 mg kinetin/L. Forty-eight to fifty-two days were required for same callus initiation and from hypocotyl was recorded in MS medium with 4mg of 2, 4 –D/L The highest biomass was recorded in callus induced from hypocotyls on MS medium supplemented with 2, 4-D (4 mg/L). The slowest induction in MS medium supplemented with 5 mg of 2, 4-D/L at 68 days. Callus induced from hypocotyls grew comparatively more rapidly than calluses from other explants. Chlorophyll loss was observed during sub culturing of the callus. The growth of white and friable callus induced from hypocotyls was significantly higher compared to that of green callus. No morphological change was noticed in white callus with subculturing on MS medium for several months. |
| Roots of Chlorophytum borivilianum (Safed musli) were regenerated by taking explants from isolated field grown matured plants. Two types explants stem disc and shoot base were used in the micropropagation. This was achieved by culturing on MS solid medium with and without hormones. Both leaf base and tuber explants gave very poor response. MS medium containing 3mg/L NAA gave more number of roots. The root proliferation was observed on full strength of MS medium after inoculation with the increasing concentration (1 to 3 mg/ml) for indole acetic acid (IAA) at 1mg/L and naphthalene acetic acid (NAA) at 3mg/L. Micropropagation is to be the effective method compared with other methods of multiplication. This protocol can be used to generate cost-effective protocol for large scale in vitro cultivation of safed musli Jakkulwar, et al [16]. |
MATERIAL AND METHODS |
i. Selection of plant material and surface serialization |
| Elite plants material selected from Shri Shail Medifarms, Vidyavihar colony, Pratap Nagar square, Nagpur- 440022 India, on the basis of higher growth and young plant leaves. The same is authenticated by Prof. Dr. L. P. Dalal, Department of Botany, J. B. College of Science, Wardha- 442001, Maharashtra. The selected explant was washed thoroughly under running tap water (30 min) and with Tween – 20 (10 min). Further it was surface disinfected with freshly prepared70% alcohol (30 sec) after this treatment explants were surface sterilized by 0.1% (w/v) aqueous solution of mercuric chloride for 5 minutes and again rinsed with washed with double distilled water. |
ii. Culture media and conditions |
| MS medium [10] supplemented with 30 g/L (w/v) sucrose and 8mg/L (w/v) phytogel was used for the experiments. The pH of the medium was adjusted to 5.8 by 1 N NaOH. The culture vials containing media were autoclaved at 121°C at 106 kPa for 20 min. All cultures were maintained at 25 ± 2°C under 16 hours photoperiod with fluorescence lighting. |
iii. Media preparation and sample inoculation |
| For shoot proliferation, MS medium was supplemented with various concentrations of Cytokine (BAP, 0 – 1.0 mg/L). Three shoot clumps (each clump of 2 – 3 shoots) were inoculated per culture flask in triplicates. In another experiment MS media supplemented with 30 gm/l agar + 0.5 mg/L BAP was also studied. |
iv. Callus induction |
| For callus induction, MS medium was supplemented with various concentration combination of Cytokine (BAP, 0-1 mg/L) and Auxine (NAA, 0-1 mg/L). Young leaf explant was vertically inoculated on MS medium. |
v. Rooting of micro-shoots |
| Regenerated shoots were transferred to MS medium containing various auxins IAA (0– 1mg/L) and heat shock also analyzed on rooting. In another 250 ml flask 10 – 15 shoot clumps were inoculated in each flask in triplicates and root development were recorded after 21 days. |
vi. Acclimatization |
| After 21 days of culture on rooting media, the plantlets were shifted to polythene bags filled with various soil mixes for hardening prior to final transfer to natural conditions. Plants with newly formed roots were carefully taken out and dipped in warm water (not hot) to remove any traces of solidified agar media. Farther plants were dipped in 1% (w/v) solution of Bavistin to prevent any fungal infection possibilities. These were carefully planted in plastic pots containing different soil mixtures having vermicompost, soil, and sand in ratio (1:1:1). After planting the plants were watered and kept under polyhouse having 80% humidity for ten days. Subsequently, plants in polyhouse were shifted to shade house with less humidity level and exposure indirect sunlight. |
OBSERVATIONS AND RESULTS |
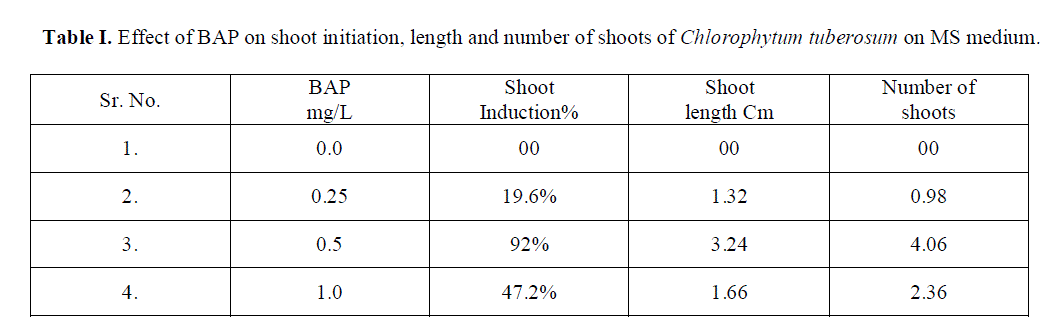
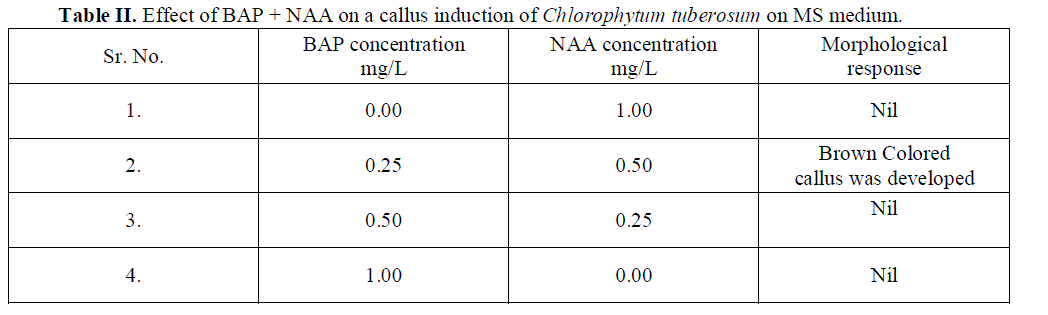
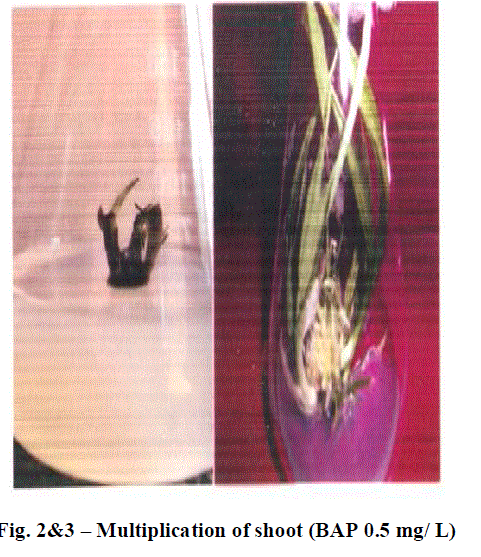
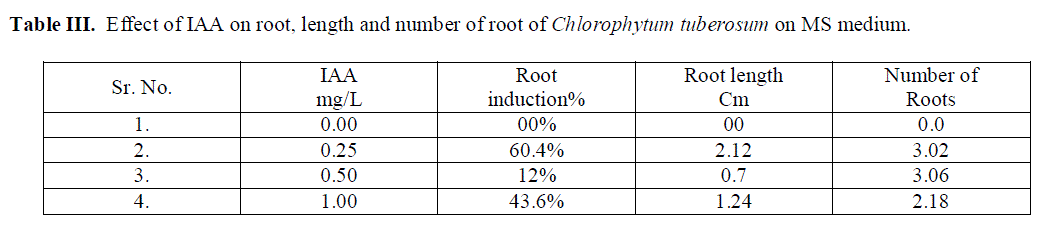
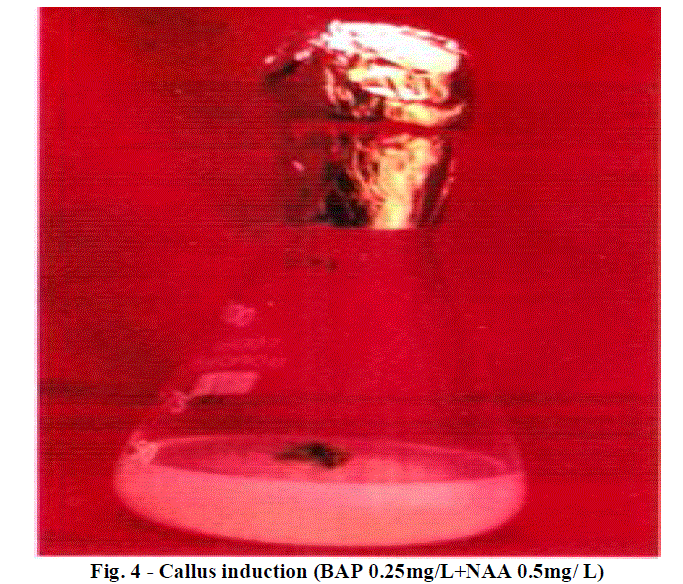
| Role of 70% ethyl alcohol and 0.1% HgCl2 as disinfecting solution is very significant in order to make explants free from any type of infection. The morphogenetic responses of shoot bud explants to BAP, BAP+ NAA, and IAA are summarized in Table 1, 2 and 3 respectively. Within 3 weeks of culture on shoot induction medium new shoots were started to proliferate, Highest mean number of shoots 4.06 units was initiated per explant, auxiliary bud was found to be able to produced shoots on MS medium + BAP 0.5 mg/L concentration(Table 1).The callus induction in C. tuberosum at 0.50 mg/L BAP and 0.25mg/L NAA was observed and maximum cell mass with brown colored callus was developed (Table 2).To induce roots in the regenerated shoots was grown in IAA with different concentrations, the beneficial effect was observed in 0.25 mg/L IAA medium, showing mean number 3.06 units of roots initiated (Table 3). Plantlets were transplanted to small plastic cups for hardening pots containing sand, soil, vermicompost (1:1:1). The transplanted plantlets were kept under shades and then transferred to normal environmental condition; through this process of acclimatization almost 76% survivals of the tissue cultured plantlets were achieved. |
OBSERVATION TABLES |
 |
 |
| With the increased supplementation of BAP from nil to 1.0 mg/L in culture medium shoot induction is accelerated almost up to four times more that initial concentration at 0.5 mg/L. Further increase in BAP shows significant reduction in shoot induction. |
 |
 |
| Role of BAP and NAA in callus induction is well known hence BAP is supplemented in medium increasing concentration form 0.00 mg/L to 1.00 mg/L in combination with NAA ranges in decreasing concentration from 1.00 mg/L to 0.00 mg/L for callus induction. Dose of BAP 0.25 mg/L and NAA 0.50 mg/L shows the brown colored callus induction. Other combinations of BAP and NAA not at all show any symptoms of callus induction. |
 |
 |
CONCLUSION |
| From the above study, one can conclude that shoot initiation of Chlorophytum tuberosum were established from shoot bud explants on MS medium supplemented with BAP (0.5 mg/L).Callus was induced from leaf explant vertically inoculated on MS medium supplemented with BAP (0.25 mg/L) + NAA (0.50 mg/L). The rooting of regenerated shoot was successfully carried out on MS media supplemented with IAA (0.50 mg/L).The aim of this study was to develop a standard protocol to initiate, multiple shoot culture of Chlorophytum tuberosum, which found to be achieved in cost effective manner. This technique is efficient for mass propagation of Chlorophytum tuberosum because it has progressively increasing medicinal importance resulting in excessive demand in market that which supposed to be full filled by this in cost effective manner. |
ACKNOWLEDGEMENT |
| The authors are grateful to Principal, New Arts Commerce and Science College, Wardha, Maharashtra, India 442001 for the laboratory facilities and for the financial support. |
References |
|